7.6: Types of Lasers
( \newcommand{\kernel}{\mathrm{null}\,}\)
There are many types of lasers: gas, solid, liquid, semiconductor, chemical, excimer, e-beam, free electron, fiber and even waveguide lasers. We classify them according to the pumping mechanism.
7.6.1 Optical Pumping
The energy to transfer the atom A from the ground state to the excited state is provided by light. The source could be another laser or an incoherent light source, such as a discharge lamp. If A is the atom in the ground state and A^{*} is the excited atom, we have \hbar \omega_{02}+A \rightarrow A^{*} \nonumber where \omega_{02} is the frequency for the transition 0 \rightarrow 2 as seen in Figure \PageIndex{7}. The Ruby laser, of which the amplifying medium consists of \mathrm{Al}_{2} \mathrm{O}_{3} with 0.05 weight percent \mathrm{Cr}_{2} \mathrm{O}_{3}, was the first laser, invented in 1960. It emits pulses of light of wavelength 694.3 \mathrm{~nm} and is optically pumped with a gas discharge lamp. Other optically pumped lasers are the YAG, glass, fiber, semiconductor and dye laser. In the dye laser the amplifier is a liquid (e.g. Rhodamine6G). It is optically pumped by an argon laser and has a huge gain width, which covers almost the complete visible wavelength range. We can select a certain wavelength by inserting a dispersive element like the Fabry-Perot cavity inside the laser cavity and rotating it at the right angle to select the desired wavelength, as explained above.
7.6.2 Electron-Collision Pump
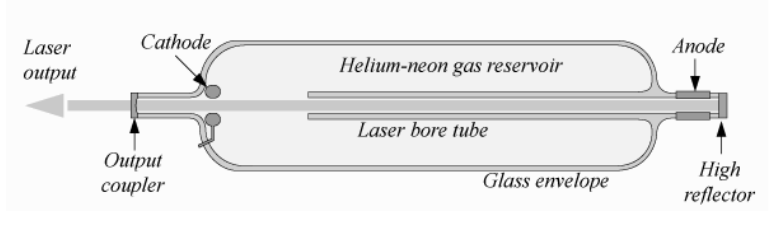
Energetic electrons are used to collide with the atoms of the amplifier, thereby transferring some of their energy: A+e\left(\mathcal{E}_{1}\right) \rightarrow A^{*}+e\left(\mathcal{E}_{2}\right), \nonumber where e\left(\mathcal{E}_{1}\right) means an electron with energy \mathcal{E}_{1} and where \mathcal{E}_{1}-\mathcal{E}_{2} is equal to \hbar \omega_{02} so that the atom is transferred from the ground state to state 2 to obtain population inversion. Examples are the HeNe, Argon, Krypton, Xenon, Nitrogen and Copper lasers. Electrons can be created by a discharge or by an electron beam.
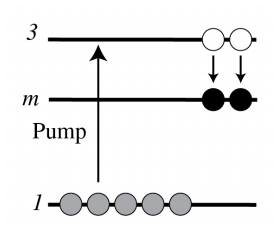
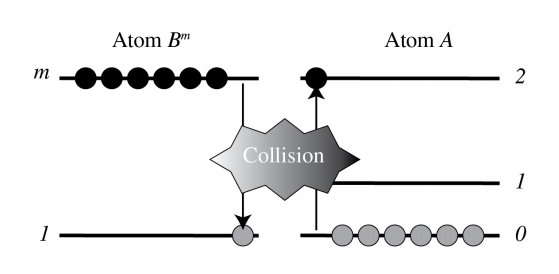
7.6.3 Atomic Collision
Let B^{m} be atom B in an excited, so-called metastable state. This means that B^{m}, although unstable, has a very long relaxation time, i.e. longer than 1 \mathrm{~ms} or so. If B^{m} collides with atom A, it transfers energy to A.
B^{m}+A \rightarrow B+A^{*}, \nonumber A^{*} is the excited state used for the stimulated emission. If \tau_{m 1} is the relaxation time of metastable state B^{m}, then \tau_{m 1} is very large and hence the spontaneous emission rate is very small. This implies that the number of metastable atoms as function of time t is given by a slowly decaying exponential function \exp \left(-t / \tau_{m 1}\right). How can one get metastable atoms? One can for example pump atom B from its ground state 1 to an excited state 3 above state \mathrm{m}, such that the spontaneous emission rate 3 \rightarrow m is large. The pumping can be done electrically or by any other means. If it is done electrically, then we have B+e\left(\mathcal{E}_{2}\right) \rightarrow B^{m}+e\left(\mathcal{E}_{1}\right), \nonumber
Examples of these types of laser are He-Ne, which emits in the red at 632 \mathrm{~nm}, \mathrm{~N}_{2} - \mathrm{CO}_{2} and He-Cd. All of these depend on atom or molecule collisions, where the atom or molecule that is mentioned first in the name is brought into the metastable state and lasing occurs at a wavelength corresponding to a level difference of the second mentioned atom or molecule. In the simplest case the metastable states are created by electrons generated by a discharge. The \mathrm{CO}_{2} laser emits at 10 \mu \mathrm{m} and can achieve huge power.
7.6.4 Chemical Pump
In some chemical reactions, a molecule is created in an excited state with population inversion. An example is: A+B_{2} \rightarrow(A B)^{*}+B \nonumber So in this case the lasing will take place for a transfer between states of molecule A B. The HF, DF, Ar-F, Cr-F, Xe-F and Xe-Cl lasers are all chemically pumped.
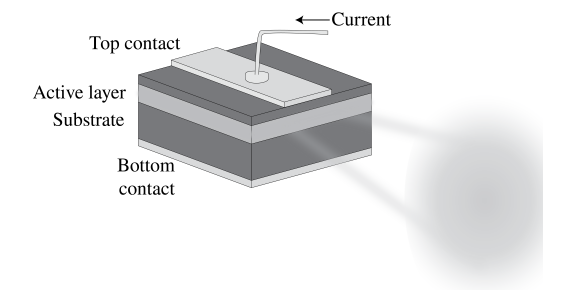
7.6.5 Semiconductor Laser
In this case pumping is done by electron current injection. It is one of the most compact lasers and yet it typically emits 20 \mathrm{~mW} of power. Transitions occur between the conduction and valence bands close to the p-n junction. Electrons from the n-layer conduction band will recombine with the holes in the p-layer. A cavity is obtained by polishing the end faces that are perpendicular to the junction to make them highly reflecting. Semiconductor lasers are produced for wavelengths from 700 \mathrm{~nm} to 30 \mu \mathrm{m} and give continuous (CW) output.