18.1: Continuous Matter or Atoms?
( \newcommand{\kernel}{\mathrm{null}\,}\)
From the time of the ancient Greeks there have been debates about the ultimate nature of matter. One of these debates is whether matter is infinitely divisible or whether it consists of fundamental building blocks that are themselves indivisible. However, it wasn’t until the late 19th century that real progress began to be made on this question.
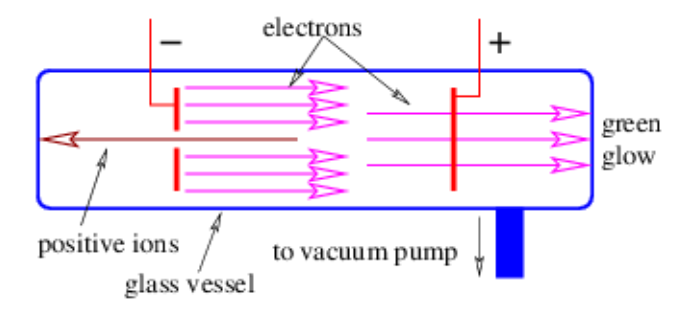
Advancements in our understanding of matter have largely been coupled to the development of machines to accelerate atomic and sub-atomic particles. The original accelerator was developed in the 19th century and is called the Crookes tube.
J. J. Thomson measured the charge to mass ratio for both electrons and positive ions in the Crookes tube in the following way: If a potential difference Δϕ is applied between the electrodes, then by energy conservation a particle of charge q starting from rest will acquire a kinetic energy moving from electrode to electrode of K=mv2/2=gΔϕ. Solving for v, we find v=(2qΔϕ/m)1/2. If a magnetic field B is then imposed normal to the electron beam after it has passed the positive electrode, the beam bends with a radius of curvature of R=mv/(qB). Since R and B are known, the charge to mass ratio can be computed by eliminating v and solving for q/m:q/m=2Δϕ/(BR)2. Thomson found that positive ions typically had charge to mass ratios several thousand times smaller than the electrons. Furthermore, the ions were positively charged, while the electrons were negatively charged. If the ions and the electrons have electrical charges equal in magnitude (plausible, since the ions are neutral atoms with at least one electron removed), the ions have to be much more massive than the electrons.
Robert Millikan made the first direct measurement of electric charge. He did this by suspending electrically charged oil drops in a known electric field against gravity. The size of an oil drop is directly measured using a microscope, leading to a calculation of its mass, and hence the gravitational force, mg. This is then balanced against the electric force, qE, leading to q=mg/E. Occasionally an oil drop loses an electron due to photoelectric emission caused by photons from an ultraviolet lamp. This disrupts the force balance, and causes the oil drop to move up or down. If the electric field is quickly adjusted, this motion can be arrested. The change in the charge can be related to the change in the electric field: Δq=mgΔ(1/E). If only a single electron is emitted, then Δq is equal to the electronic charge.
Between the work of Thomson and Millikan, the masses and the charges of sub-atomic particles were accurately measured for the first time. Ironically, this work also showed that the “atom”, which means “indivisible” in Greek, in fact isn’t. Atoms consist of positive charges with large mass, or protons, in conjunction with low mass electrons of negative charge. Electrons and protons have opposite charges, so they attract each other to form atoms in this picture.
Geiger and Marsden did an experiment that strongly suggested that atoms consist of very small, positively charged atomic nuclei, surrounded by a cloud of circling, negatively charged electrons. This is called the Rutherford model of the atom after Ernest Rutherford.
Chadwick completed our picture of the atom with the discovery of a neutral particle of mass comparable to the proton, called the neutron. The neutron is a constituent of the atomic nucleus along with the proton. The number of protons in a nucleus is denoted Z while the number of neutrons is N. We define A=Z+N to be the total number of nucleons (protons plus neutrons). The parameter Z is often called the atomic number while A is called the atomic mass number.
Marie and Pierre Curie and Henri Becquerel were the first to discover a more fundamental divisibility of atoms in the form of the radioactive decay, though the implications of their results did not become clear until much later. Radioactive decay of atomic nuclei comes in three common forms: alpha, beta, and gamma decay. Alpha decay is the spontaneous emission of a helium-4 nucleus, called an alpha particle by a heavy nucleus such as uranium or radium. The alpha particle consists of two protons and two neutrons, so the emission decreases both Z and N by 2. Beta decay is the emission of an electron or its antiparticle, the positron, by a nucleus, with an accompanying change in the electric charge of the nucleus. For electron emission Z increases by 1 while N decreases by 1. The opposite occurs for positron emission. Gamma decay is the emission of a high energy photon by a nucleus. The values of Z and N remain unchanged. The energy released by these decays is typically of order a few million electron volts.
Of the three forms of decay, beta decay is the most interesting, since it involves the transformation of one sub-atomic particle into another. In the case of neutron decay, a neutron is converted into a proton, an electron, and an antineutrino. For proton decay, a proton becomes a neutron, a positron, and a neutrino. (Only the neutron form occurs for an isolated particle. However, the energetics inside atomic nuclei can result in either form, depending on the nucleus in question.) The neutrino is one of the great theoretical predictions of modern physics. Careful studies of beta decay, which at the time was thought to result only in the emission of a proton and an electron for the neutron form of the reaction, showed apparent non-conservation of energy and angular momentum. Rather than accept this rather unpalatable conclusion, Wolfgang Pauli proposed that a third particle named a neutrino, or little neutral particle, is emitted in the decay, thus accounting for the missing energy and angular momentum. The presumed electrical neutrality of the particle explained the difficulty of detecting it. Over 25 years passed before Frederick Reines and Clyde Cowan from Los Alamos observed this elusive particle.
The three forms of radioactive decay are associated with three of the four known fundamental forces of nature. Gamma decay is electromagnetic in nature, while alpha decay involves the breaking of bonds produced by the nuclear or strong force. Beta decay is a manifestation of the so-called weak force. (The fourth force is gravity, which plays a negligible role on the sub-atomic scale, as far as we know.)
Beta decay gives us a strong hint that even particles such as protons and neutrons, which make up atomic nuclei, are not “atomic” in the sense of the original Greek, since neutrons can change into protons in beta decay and vice versa. We now have excellent evidence that protons, neutrons, and many other sub-nuclear particles are made up of particles called quarks. Quarks and electrons are currently thought to be fundamental in that they are supposedly indivisible, and are hence the true “atoms” of the universe. However, who knows, perhaps someday we will discover that they too are composed of even more fundamental constituents!


