24.2: Heat Engines
( \newcommand{\kernel}{\mathrm{null}\,}\)
Heat engines typically operate by heating and cooling a volume of gas and by compressing or expanding the gas. If these operations are done in a particular order, internal energy can be converted to useful work. We therefore seek to understand how an ideal gas reacts to the addition and subtraction of internal energy and to the change in the volume of the gas.
The equation for the entropy of an ideal gas and the ideal gas law contain the information we need. The entropy of an ideal gas is a function of its internal energy E and its volume V . (We assume that the number of molecules in the gas remains fixed.) Thus, a small change ΔS in the entropy can be related to small changes in the energy and volume as follows:
ΔS=∂S∂EΔE+∂S∂VΔV
We know that ∂S/∂E=1/T. Using equation (24.12) we can similarly calculate ∂S/∂V=NkB/V=p/T, where the ideal gas law is used in the last step to eliminate NkB in favor of p. Substituting these into the above equation, multiplying by T, and solving for pΔV results in
pΔV=ΔW=TΔS−ΔE (work by ideal gas)
where we have recognized pΔV = ΔW to be the work done by the gas on the piston.
We are now in a position to investigate the conversion of internal energy to useful work. If the gas is allowed to push the piston out in a reversible adiabatic manner, then ΔS = 0 and energy is converted with 100% efficiency from internal form to work. This work could in principle be used to run an electric generator, stretch springs, power an automobile, etc.
Unfortunately, a piston in a cylinder that can only extract energy during a single expansion wouldn’t be very useful — it would be like an automobile engine that only worked for half a turn of the crankshaft and then had to be replaced! If the piston is simply pushed back into the cylinder, then the macroscopic energy gained from the initial expansion would be converted back into internal energy of the gas, resulting in zero net creation of useful work.
The trick to obtaining non-zero useful work from the expansion and contraction of a gas is to add heat to the gas before the expansion and extract heat from it before the recompression. This makes the gas cooler in the compression than in the expansion. The pressure is therefore less in the compression and the work needed to compress the gas is less than that produced in the expansion.
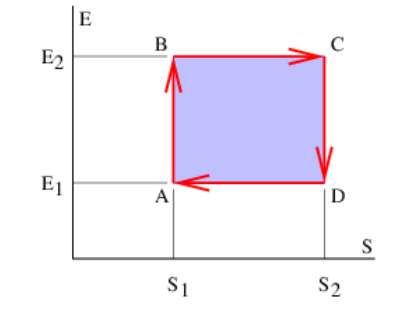
Figure 24.2.7: shows a particular way of executing a complete cycle of expansion and compression of the gas that results in a net conversion of internal energy to useful work. Assuming that the gas has initial entropy and internal energy S1 and E1 at point A in Figure 24.2.7:, the gas is first compressed in reversible adiabatic fashion to point B. The entropy doesn’t change in this compression but the internal energy increases from E1 to E2. The work done by the gas is negative and equals WAB=E1−E2.
The gas then is allowed to slowly expand (so that the expansion is reversible), moving from point B to point C in Figure 24.2.7: in such a way that its internal energy doesn’t change. From equation (???) we see that WBC=T2(S2−S1) for this segment of the expansion. However, heat must be added to the gas equal in amount to the work done in order to keep the internal energy fixed: Q2=T2( S2−S1).
From point C to point D the gas expands further but in this segment the expansion is reversible adiabatic so that the entropy change is again zero. Thus, WCD=E2−E1.
Finally, the gas is slowly compressed from point D to point A in a constant internal energy process. Keeping the internal energy fixed means that the (negative) work done by the gas in this segment is WDA=T1(S1−S2). Furthermore, heat equal to the work done on the gas by the piston must be removed from the gas in order to keep the internal energy constant: Q1=−WDA=T1( S2−S1). The net work done by the gas over the full cycle is obtained by adding up the contributions from each segment:
W=WAB+WBC+WCD+WDA=(E1−E2)+T2(S2−S1)+(E2−E1)+T1(S1−S2)=(T2−T1)(S2−S1) (Carnot cycle)
The energy source for this work is internal energy at temperature T2. As demanded by energy conservation, W=Q2−Q1. The fraction of the internal energy Q2 that is converted to useful work in this cycle is
ϵ=WQ2=(T2−T1)(S2−S1)T2(S2−S1)=1−T1T2( thermodynamic efficiency )
This quantity ϵ is called the thermodynamic efficiency of the heat engine. Notice that it depends only on the ratio of the lower and upper temperatures, expressed in absolute or Kelvin form. The smaller this ratio, the larger the thermodynamic efficiency.
Heat engines normally work via repeated cycling around some loop such as described above. The particular cycle we have discussed is called the Carnot cycle after the 19th century French scientist Sadi Carnot. Heat is accepted from a high temperature heat source, created, for example, by burning coal in a power plant. Excess heat is disposed of in the atmosphere or in some source of running water such as a river. Notice that the ability to get rid of excess heat at low temperature is as important to a heat engine as the supply of heat at a high temperature.
Many cycles for converting heat to work are possible — these are represented by different closed trajectories in the S-E plane. However, the Carnot cycle is special for two reasons: First, all heat absorbed by the system is absorbed at a single temperature T2, and all heat rejected from the system is rejected at a single temperature T1. This allows the expression of the efficiency simply in terms of the two temperatures. Second, the Carnot cycle is reversible, which means that no net entropy is generated.
A Carnot engine running backwards acts as a refrigerator. Heat ΔQ1 is extracted at temperature T1 from the box being cooled with the aid of externally supplied work W. An amount of heat Q2=W+Q1 is then transferred to the environment at temperature T2>T1 . . Equation (???) gives the ratio of W to Q2 in this case, as well as when the heat engine is run in the forward direction. This may be verified by tracing the cycle in Figure 24.2.7: in reverse.
In analyzing heat engines and refrigerators it is generally easier to go back to basic principles than it is to use equations such as (???) and (???). In particular, for a Carnot engine in which heat Q2 is being extracted from the high temperature reservoir (T2) and heat Q1 is being added to the low temperature reservoir (T1), conservation of energy says that the useful work extracted is W=Q2−Q1, and that the total combined entropy change in the warm and cold reservoirs is
ΔS=−Q2/T2+Q1/T1=0.. (Note that the reservoir providing energy has a minus sign, while the reservoir accepting energy has a plus sign.) Given these two relationships, any two of Q1, Q2, and W can be determined if the third is known. For a refrigerator, the higher temperature reservoir accepts energy while the lower temperature reservoir (generally the interior of the refrigerator) and the work term provide energy. This changes the signs of all three energy flows. If the machine is not a perfectly efficient Carnot engine, then ΔS>0 whether the machine is a heat engine or a refrigerator, and one deals with inequalities rather than equalities.


