1.4: Electromagnetic Spectrum
( \newcommand{\kernel}{\mathrm{null}\,}\)
By the end of this section, you will be able to:
- Define electromagnetic spectrum
- Identify ranges in the electromagnetic spectrum corresponding to different physical phenomena.
- Identify ranges in the radio-frequency spectrum and their applications.
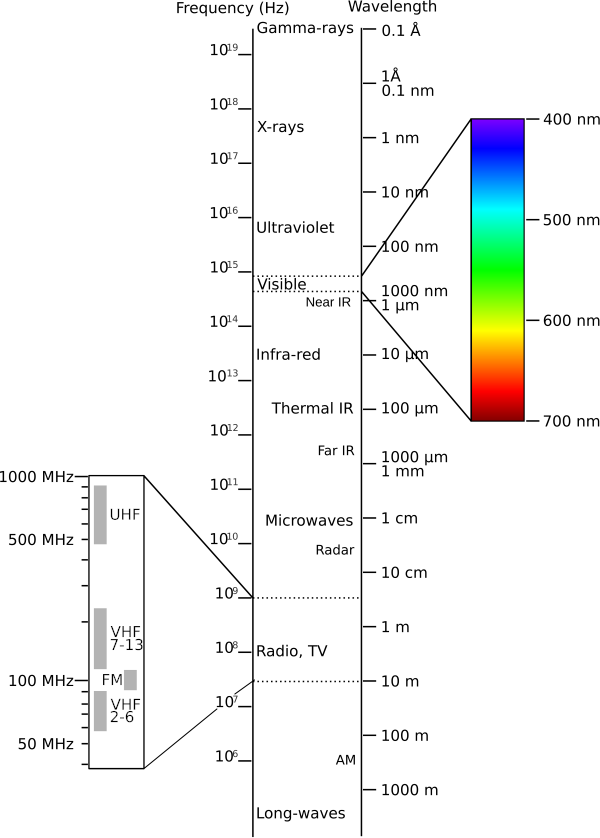
Electromagnetic waves are oscillating disturbances in the electromagnetic field. The frequency of the wave is the number of oscillations per second and is given in units of hertz (1 Hz = 1 oscillation per second). Electromagnetic fields exist at frequencies from 0 Hz to at least 1020 Hz – that’s at least 20 orders of magnitude! At 0 Hz, electromagnetics consists of two distinct disciplines: electrostatics, concerned with electric fields, and magnetostatics, concerned with magnetic fields. At higher frequencies, electric and magnetic fields interact to form propagating waves. Waves having frequencies within certain ranges are given names based on how they manifest as physical phenomena. These names are (in order of increasing frequency) radio, infrared (IR), optical (also known as “light”), ultraviolet (UV), X-rays, and gamma rays (γ-rays). See Table 1.4.1 for the corresponding frequency ranges.
The term electromagnetic spectrum refers to the various forms of electromagnetic phenomena that exist over the continuum of frequencies [1].
The speed (properly known as “phase velocity”) at which electromagnetic fields propagate in free space is given the symbol c, and has the value of 299,792,458 m/s (exactly) or approximately 3.00×108 m/s. This value is often referred to as the “speed of light.” While it is certainly the speed of light in free space, it is also the speed of any electromagnetic wave in free space. Given frequency f, wavelength is given by the expression
λ=cf⏟in free space
A sinusoidal wave repeats itself in space over a distance of the wavelength, as illustrated in Figure 1.4.1. According to Eq. ???, the wavelength and frequency are inversely related so that as one increases, the other decreases. To a good approximation, the wavelength in meters can then be calculated as 300 (in Mm) divided by the frequency in MHz.
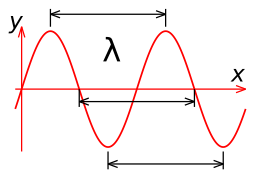
Table 1.4.1 shows the free space wavelengths associated with each of the regions of the electromagnetic spectrum.
| Regime | Frequency Range | Wavelength Range |
|---|---|---|
| γ-Ray | >3×1019Hz | < 0.01 nm |
| X-Ray | 3×1016Hz–3×1019Hz | 10–0.01 nm |
| Ultraviolet (UV) | 2.5×1015–3×1016Hz | 120–10 nm |
| Optical | 4.3×1014–2.5×1015Hz | 700–120 nm |
| Infrared (IR) | 300GHz–4.3×1014Hz | 1 mm – 700 nm |
| Radio | 3kHz–300GHz | 100 km – 1 mm |
This book presents a version of electromagnetic theory that is based on classical physics. This approach works well for most practical problems. However, at very high frequencies, wavelengths become small enough that quantum mechanical effects may be important. This effect usually happens in the X-ray band and above. In some applications, these effects become important at frequencies as low as the optical, IR, or radio bands. (A prime example is the photoelectric effect. [3]) Thus, caution is required when applying the classical version of the electromagnetic theory presented here, especially at these higher frequencies.
The theory presented in this book applies to static fields (0 Hz) along with radio, infrared, and light waves and, to a lesser extent, to ultraviolet waves, X-rays, and gamma rays. Certain phenomena in these frequency ranges – in particular, quantum mechanical effects – are not addressed in this book.
The radio-frequency (RF) portion of the electromagnetic spectrum alone spans 12 orders of magnitude in frequency (and wavelength), and so, not surprisingly, exhibits a broad range of phenomena (Figure 1.4.2). For this reason, the radio spectrum is further subdivided into bands as shown in Table 1.4.2 [4]. Also shown in Table 1.4.2 are commonly used band identification acronyms and some typical applications. Amateur Radio operators can use portions of the radio bands starting as low as the Low Frequency (LF) band through the Extremely High Frequency (EHF) band and beyond, depending on their license class. However, in practice, activity for Amateur Radio tends to occur primarily on the High Frequency (HF), Very High Frequency (VHF), and Ultra High Frequency (UHF) bands.
Similarly, the optical band is partitioned into the familiar "rainbow" of red through violet, as shown in Figure 1.4.2 and Table 1.4.3. Other portions of the spectrum are sometimes similarly subdivided in certain applications.
| Band | Frequencies | Wavelengths | Typical Applications |
|---|---|---|---|
| THF (Tremendously High Frequency) |
300–3000 GHz (0.3–3 THz) |
1–0.1 mm | Short-range communication, Imaging, Spectroscopy |
| EHF (Extremely High Frequency) |
30–300 GHz | 10–1 mm | 60 GHz WLAN, Point-to-point data links |
| SHF (Super High Frequency) |
3–30 GHz | 10–1 cm | Terrestrial & Satellite data links, Radar |
| UHF (Ultra High Frequency) |
300–3000 MHz | 1–0.1 m | TV broadcasting, Cellular, WLAN |
| VHF (Very High Frequency) |
30–300 MHz | 10–1 m | FM & TV broadcasting, LMR |
| HF (High Frequency) |
3–30 MHz | 100–10 m | Global terrestrial comm., CB Radio |
| MF (Medium Frequency) |
300–3000 kHz | 1000–100 m | AM broadcasting |
| LF (Low Frequency) |
30–300 kHz | 10–1 km | Navigation, RFID |
| VLF (Very Low Frequency) |
3–30 kHz | 100–10 km | Navigation |
| ULF (Ultra Low Frequency) |
300–3000 Hz | 1000–100 km | Underground communication |
| SLF (Super Low Frequency) |
30–300 Hz | 10000–1000 km | Underwater communication |
| ELF (Extremely Low Frequency) |
3–30 Hz | 100000–10000 km | Underwater communication, Lightning |
| Band | Frequencies | Wavelengths |
|---|---|---|
| Violet | 668–789 THz | 450–380 nm |
| Blue | 606–668 THz | 495–450 nm |
| Green | 526–606 THz | 570–495 nm |
| Yellow | 508–526 THz | 590–570 nm |
| Orange | 484–508 THz | 620–590 nm |
| Red | 400–484 THz | 750–620 nm |
References
- Wikipedia contributors. Electromagnetic spectrum [Internet]. Wikipedia, The Free Encyclopedia.
- Wikimedia Commons contributors. File:Sine wavelength.svg [Internet]. Wikimedia Commons. (CC BY-SA 3.0, Richard F. Lyon)
- Wikipedia contributors. Photoelectric effect [Internet]. Wikipedia, The Free Encyclopedia.
- Wikipedia contributors. Radio spectrum [Internet]. Wikipedia, The Free Encyclopedia.
- Wikimedia Commons contributors. File:Electromagnetic-Spectrum.svg [Internet]. Wikimedia Commons. (CC BY-SA 3.0, V. Blacus)
Technician Exam Questions
The Radio Spectrum
Relevant exam questions include: T3B08‒10, T5A06, T5A12, T5C06.
Wavelength
Relevant exam questions include: T3B04‒07, T3B11.

