10.4: Applications of Nuclear Physics
- Last updated
- Save as PDF
- Page ID
- 17140
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Example \(\PageIndex{1}\):
The nuclear medicine whole body bone scan is generally used in evaluations of various bone related pathology, such as for bone pain, stress fracture, nonmalignant bone lesions, bone infections, or the spread of cancer to the bone.
Radiation therapy involves the application of ionizing radiation to treat conditions such as hyperthyroidism, thyroid cancer, and blood disorders. Radiation therapy is particularly effective as a treatment of a number of types of cancer if they are localized to one area of the body. It may also be used as part of curative therapy, to prevent tumor recurrence after surgery, or to remove a primary malignant tumor. Radiation therapy is synergistic with chemotherapy and has been used before, during, and after chemotherapy in susceptible cancers.
Ionizing radiation works by damaging the DNA of exposed tissue, leading to cellular death. When external beam therapy is used, shaped radiation beams are aimed from several angles of exposure to intersect at the tumor, providing a much larger absorbed dose there than in the surrounding, healthy tissue.
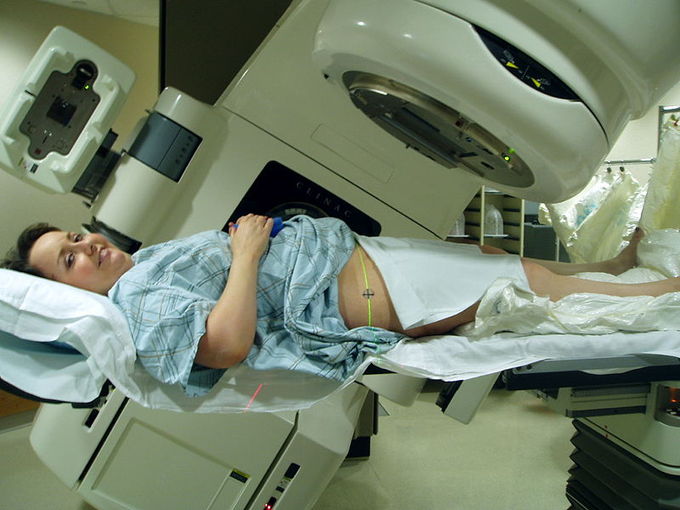
External Beam Therapy: Radiation therapy of the pelvis. Lasers and a mold under the legs are used for precise positioning
Brachytherapy is another form of radiation therapy, in which a therapeutic radioisotope is injected into the body to chemically localize to the tissue that requires destruction . A key feature of brachytherapy is that the irradiation affects only a very localized area around the radiation sources. Exposure to radiation of healthy tissues further away from the sources is therefore reduced in this technique.
Radiation therapy is in itself painless. Many low-dose palliative treatments (for example, radiation therapy targeting bony metastases) cause minimal or no side effects, although short-term pain flare-ups can be experienced in the days following treatment due to edemas compressing nerves in the treated area. Higher doses can cause varying side effects during treatment (acute), in the months or years following treatment (long-term), or after re-treatment (cumulative). The nature, severity, and longevity of side effects depend on the organs that receive the radiation, the treatment itself (type of radiation, dose, fractionation, concurrent chemotherapy), and the individual patient.
Dosimetry
Radiation dosimetry is the measurement and calculation of the absorbed dose resulting from the exposure to ionizing radiation.
learning objectives
- Explain difference between absorbed dose and dose equivalent.
Radiation dosimetry is the measurement and calculation of the absorbed dose in matter and tissue resulting from the exposure to indirect and direct ionizing radiation.
Measuring Radiation
There are several ways of measuring the dose of ionizing radiation. Workers who come in contact with radioactive substances or who may be exposed to radiation routinely carry personal dosimeters . In the United States, these dosimeters usually contain materials that can be used in thermoluminescent dosimetry or optically stimulated luminescence. Outside the United States, the most widely used type of personal dosimeter is the film badge dosimeter, which uses photographic emulsions that are sensitive to ionizing radiation. The equipment used in radiotherapy (a linear particle accelerator in external beam therapy) is routinely calibrated using ionization chambers or the new and more accurate diode technology. Internal dosimetry is used to evaluate the intake of particles inside a human being.
Ionization Chamber: This ionization chamber was used in the South Atlantic Anomaly Probe project.
Dose is reported in grays (Gy) for absorbed doses or sieverts (Sv) for dose equivalents, where 1 Gy or 1 Sv is equal to 1 joule per kilogram. Non-SI units are still prevalent as well: absorbed dose is often reported in rads and dose equivalent in rems. By definition, 1 Gy = 100 rad, and 1 Sv = 100 rem.
Biological Effects
The distinction between absorbed dose (Gy/rad) and dose equivalent (Sv/rem) is based upon the biological effects. The weighting factor (wr) and tissue/organ weighting factor (WT) have been established. They compare the relative biological effects of various types of radiation and the susceptibility of different organs.
The weighting factor for the whole body is 1, such that 1 Gy of radiation delivered to the whole body is equal to one sievert. Therefore, the WT for all organs in the whole body must sum to 1.
By definition, X-rays and gamma rays have a wr of unity, such that 1 Gy = 1 Sv (for whole-body irradiation). Values of wr are as high as 20 for alpha particles and neutrons. That is to say, for the same absorbed dose in Gy, alpha particles are 20 times as biologically potent as x-rays or gamma rays.
Dose is a measure of deposited dose and therefore can never decrease: removal of a radioactive source can reduce only the rate of increase of absorbed dose — never the total absorbed dose.
Biological Effects of Radiation
Ionizing radiation is generally harmful, even potentially lethal, to living organisms.
learning objectives
- Describe effects of ionizing radiation on living organisms.
Example \(\PageIndex{2}\):
The Radium Girls were female factory workers who contracted radiation poisoning from painting watch dials with glow-in-the-dark paint at the United States Radium factory in Orange, New Jersey around 1917. The women, who had been told the paint was harmless, ingested deadly amounts of radium by licking their paintbrushes to give them a fine point; some also painted their fingernails and teeth with the glowing substance.
Ionizing radiation is generally harmful, even potentially lethal, to living organisms. Although radiation was discovered in the late 19th century, the dangers of radioactivity and of radiation were not immediately recognized. The acute effects of radiation were first observed in the use of x-rays when Wilhelm Röntgen intentionally subjected his fingers to x-rays in 1895. The genetic effects of radiation, including the effects on cancer risk, were recognized much later. In 1927, Hermann Joseph Muller published research showing genetic effects.
Some effects of ionizing radiation on human health are stochastic, meaning that their probability of occurrence increases with dose, while the severity is independent of dose. Radiation-induced cancer, teratogenesis, cognitive decline, and heart disease are all examples of stochastic effects. Other conditions, such as radiation burns, acute radiation syndrome, chronic radiation syndrome, and radiation-induced thyroiditis are deterministic, meaning they reliably occur above a threshold dose and their severity increases with dose. Deterministic effects are not necessarily more or less serious than stochastic effects; either can ultimately lead to damage ranging from a temporary nuisance to death.

Radium Girls: Radium dial painters working in a factory
Quantitative data on the effects of ionizing radiation on human health are relatively limited compared to other medical conditions because of the low number of cases to date and because of the stochastic nature of some of the effects. Stochastic effects can only be measured through large epidemiological studies in which enough data have been collected to remove confounding factors such as smoking habits and other lifestyle factors. The richest source of high-quality data is the study of Japanese atomic bomb survivors.
Two pathways of exposure to ionizing radiation exist. In the case of external exposure, the radioactive source is outside (and remains outside) the exposed organism. Examples of external exposure include a nuclear worker whose hands have been dirtied with radioactive dust or a person who places a sealed radioactive source in his pocket. External exposure is relatively easy to estimate, and the irradiated organism does not become radioactive, except if the radiation is an intense neutron beam that causes activation. In the case of internal exposure, the radioactive material enters the organism, and the radioactive atoms become incorporated into the organism. This can occur through inhalation, ingestion, or injection. Examples of internal exposure include potassium-40 present within a normal person or the ingestion of a soluble radioactive substance, such as strontium-89 in cows’ milk. When radioactive compounds enter the human body, the effects are different from those resulting from exposure to an external radiation source. Especially in the case of alpha radiation, which normally does not penetrate the skin, the exposure can be much more damaging after ingestion or inhalation.
Therapeutic Uses of Radiation
Radiation therapy uses ionizing radiation to treat conditions such as hyperthyroidism, cancer, and blood disorders.
learning objectives
- Explain difference between external beam radiotherapy and brachytherapy.
Radiation therapy involves the application of ionizing radiation to treat conditions such as hyperthyroidism, thyroid cancer, and blood disorders. Radiation therapy is particularly effective as a treatment of a number of types of cancer if they are localized to one area of the body. It may also be used as part of curative therapy, to prevent tumor recurrence after surgery, or to remove a primary malignant tumor. Radiation therapy is synergistic with chemotherapy and has been used before, during, and after chemotherapy in susceptible cancers.
Ionizing radiation works by damaging the DNA of exposed tissue, leading to cellular death. When external beam therapy is used, shaped radiation beams are aimed from several angles of exposure to intersect at the tumor, providing a much larger absorbed dose there than in the surrounding, healthy tissue .

External Beam Therapy: Radiation therapy of the pelvis. Lasers and a mold under the legs are used for precise positioning
Brachytherapy is another form of radiation therapy, in which a therapeutic radioisotope is injected into the body to chemically localize to the tissue that requires destruction . A key feature of brachytherapy is that the irradiation affects only a very localized area around the radiation sources. Exposure to radiation of healthy tissues further away from the sources is therefore reduced in this technique.
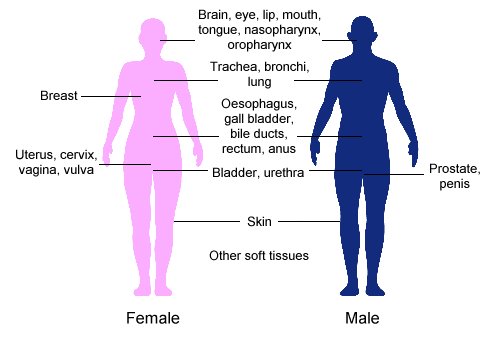
Clinical Applications of Brachytherapy: Body sites in which brachytherapy can be used to treat cancer
Radiation therapy is in itself painless. Many low-dose palliative treatments (for example, radiation therapy targeting bony metastases) cause minimal or no side effects, although short-term pain flare-ups can be experienced in the days following treatment due to edemas compressing nerves in the treated area. Higher doses can cause varying side effects during treatment (acute), in the months or years following treatment (long-term), or after re-treatment (cumulative). The nature, severity, and longevity of side effects depend on the organs that receive the radiation, the treatment itself (type of radiation, dose, fractionation, concurrent chemotherapy), and the individual patient.
Radiation from Food
Food irradiation is a process of treating a food to a specific dosage of ionizing radiation for a predefined length of time.
learning objectives
- Explain how food irradiation is performed, commenting on its purpose and safety
Food irradiation is a process of treating a food to a specific dosage of ionizing radiation for a predefined length of time. This process slows or halts spoilage that is due to the growth of pathogens. Food irradiation is currently permitted by over 50 countries, and the volume of food treated is estimated to exceed 500,000 metric tons annually worldwide. Irradiated food is sold in regular stores, often in specially marked packages .

Radura Logo: The Radura logo, required by U.S. Food and Drug Administration regulations to show a food has been treated with ionizing radiation
By irradiating food, depending on the dose, some or all of the microorganisms, bacteria, viruses, and insects present are killed. This prolongs the shelf-life of the food in cases where pathogenic spoilage is the limiting factor. Some foods, e.g., herbs and spices, are irradiated at sufficient doses (five kilograys or more) to reduce the microbial counts by several orders of magnitude. Such ingredients do not carry spoilage or pathogen microorganisms into the final product. It has also been shown that irradiation can delay the ripening of fruits and the sprouting of vegetables.
Food irradiation using cobalt-60 is the preferred method by most processors. This is because the deep penetration of gamma rays allows for the treatment of entire industrial pallets or totes at once, which reduces the need for material handling. A pallet or tote is typically exposed for several minutes to several hours, depending on the dose. Radioactive material must be monitored and carefully stored to shield workers and the environment from its gamma rays. During operation this is achieved using concrete shields. With most designs the radioisotope can be lowered into a water-filled source storage pool to allow maintenance personnel to enter the radiation shield. In this mode the water in the pool absorbs the radiation.
X-ray irradiators are considered an alternative to isotope-based irradiation systems. X-rays are generated by colliding accelerated electrons with a dense material (the target), such as tantalum or tungsten, in a process known as bremsstrahlung-conversion. X-ray irradiators are scalable and have deep penetration comparable to Co-60, with the added benefit that the electronic source stops radiating when switched off. They also permit dose uniformity, but these systems generally have low energetic efficiency during the conversion of electron energy to photon radiation, so they require much more electrical energy than other systems. X-ray systems also rely on concrete shields to protect the environment and workers from radiation.
Irradiated food does not become radioactive, since the particles that transmit radiation are not themselves radioactive. Still, there is some controversy in the application of irradiation due to its novelty, the association with the nuclear industry, and the potential for the chemical changes to be different than the chemical changes due to heating food (since ionizing radiation produces a higher energy transfer per collision than conventional radiant heat).
Tracers
A radioactive tracer is a chemical compound in which one or more atoms have been replaced by a radioisotope.
learning objectives
- Explain structure and use of radioactive tracers
A radioactive tracer is a chemical compound in which one or more atoms have been replaced by a radioisotope. By virtue of its consequent radioactive decay, this compound can be used to explore the mechanism of chemical reactions by tracing the path that the radioisotope follows from reactants to products.
The underlying principle in the creation of a radioactive tracer is that an atom in a chemical compound is replaced by another atom of the same chemical element. In a tracer, this substituting atom is a radioactive isotope. This process is often called radioactive labeling. Radioactive decay is much more energetic than chemical reactions. Therefore, the radioactive isotope can be present in low concentration and its presence still detected by sensitive radiation detectors such as Geiger counters and scintillation counters.

Geiger Counter: Image of a Geiger counter with pancake-type probe
There are two main ways in which radioactive tracers are used:
When a labeled chemical compound undergoes chemical reactions, one or more of the products will contain the radioactive label. Analysis of what happens to the radioactive isotope provides detailed information about the mechanism of the chemical reaction.
A radioactive compound can be introduced into a living organism. The radio-isotope provides a way to build an image showing how that compound and its reaction products are distributed around the organism.
All the commonly used radioisotopes (Tritium \(\mathrm{(^3H), ^{11}C, ^{13}N, ^{15}O, ^{18}F, ^{32}P, ^{35}S, ^{99m}Tc,}\) and \(\mathrm{^{123}I}\)) have short half-lives. They do not occur in nature and are produced through nuclear reactions.

Iodine; 123 Radioisotope: Lead container containing iodine-123 radioisotope
Nuclear Fusion
In nuclear fusion two or more atomic nuclei collide at very high speed and join, forming a new nucleus.
learning objectives
- Analyze possibility of the use of nuclear fusion for the production of electricity.
Example \(\PageIndex{3}\):
The sun is a main-sequence star and therefore generates its energy through nuclear fusion of hydrogen nuclei into helium. In its core, the sun fuses 620 million metric tons of hydrogen each second.
Nuclear fusion is a nuclear reaction in which two or more atomic nuclei collide at very high speed and join to form a new type of atomic nucleus. During this process, matter is not conserved because some of the mass of the fusing nuclei is converted into energy.
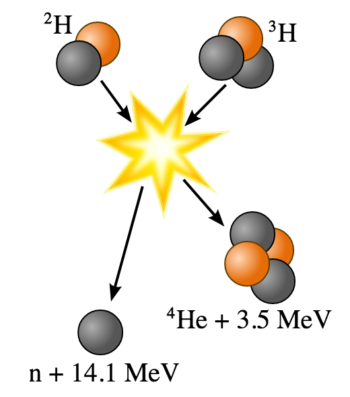
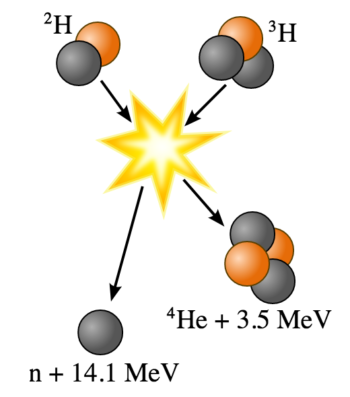
Fusion of Deuterium with Tritium: Fusion of deuterium with tritium creating helium-4, freeing a neutron, and releasing 17.59 MeV of energy; some mass changes form to appear as the kinetic energy of the products
Fission and Fusion
Describes the difference between fission and fusion
Fusion reactions of light elements power the stars and produce virtually all elements in a process called nucleosynthesis. The fusion of lighter elements in stars releases energy and mass. For example, in the fusion of two hydrogen nuclei to form helium, 0.7 percent of the mass is carried away from the system in the form of kinetic energy or other forms of energy (such as electromagnetic radiation).
It takes considerable energy to force nuclei to fuse, even nuclei of the lightest element, hydrogen. This is because all nuclei have a positive charge due to their protons, and since like charges repel, nuclei strongly resist being put close together. Accelerated to high speeds, they can overcome this electrostatic repulsion and be forced close enough for the attractive nuclear force to be sufficiently strong to achieve fusion. The fusion of lighter nuclei, which creates a heavier nucleus and often a free neutron or proton, generally releases more energy than it takes to force the nuclei together. This is an exothermic process that can produce self-sustaining reactions.
Research into controlled fusion, with the aim of producing fusion power for the production of electricity, has been conducted for over 60 years. It has been accompanied by extreme scientific and technological difficulties, but it has resulted in progress. At present, controlled fusion reactions have been unable to produce self-sustaining controlled fusion reactions. Researchers are working on a reactor that theoretically will deliver 10 times more fusion energy than the amount needed to heat up plasma to required temperatures. Workable designs of this reactor were originally scheduled to be operational in 2018; however, this has been delayed, and a new date has not been released.
Nuclear Fission in Reactors
Nuclear reactors convert the thermal energy released from nuclear fission into electricity.
learning objectives
- Explain how nuclear chain reactions can be controlled.
Example \(\PageIndex{4}\):
Some serious nuclear and radiation accidents have occurred. In 2011, three of the reactors at Fukushima I overheated, causing meltdowns that eventually led to explosions, which released large amounts of radioactive material into the air.
Nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller (lighter) nuclei. This reaction often produces free neutrons and photons (in the form of gamma rays) and releases a very large amount of energy, even by the standards of radioactive decay . The two nuclei produced are most often of comparable but slightly different sizes, typically with a mass ratio of products of about 3 to 2, for common fissile nuclides.
Nuclear Fission Reaction: An induced nuclear fission event. A neutron is absorbed by the nucleus of a uranium-235 atom, which in turn splits into fast-moving lighter elements (fission products) and free neutrons
For example, when a large fissile atomic nucleus such as uranium-235 or plutonium-239 absorbs a neutron, it may undergo nuclear fission. The heavy nucleus splits into two or more lighter nuclei (the fission products), releasing kinetic energy, gamma radiation, and free neutrons. A portion of these neutrons may later be absorbed by other fissile atoms and trigger further fission events, which release more neutrons, and so on. This is known as a nuclear chain reaction.
Just as conventional power stations generate electricity by harnessing the thermal energy released from burning fossil fuels, the thermal energy released from nuclear fission can be converted in electricity by nuclear reactors. A nuclear chain reaction can be controlled by using neutron poisons and neutron moderators to change the percentage of neutrons that will go on to cause more fissions. Nuclear reactors generally have automatic and manual systems to shut the fission reaction down if unsafe conditions are detected.
The reactor core generates heat in a number of ways. The kinetic energy of fission products is converted to thermal energy when these nuclei collide with nearby atoms. Some of the gamma rays produced during fission are absorbed by the reactor, and their energy is converted to heat. Heat is produced by the radioactive decay of fission products and materials that have been activated by neutron absorption. This decay heat source will remain for some time even after the reactor is shut down.
A nuclear reactor coolant — usually water, but sometimes a gas, liquid metal, or molten salt — is circulated past the reactor core to absorb the heat that it generates. The heat is carried away from the reactor and is then used to generate steam.
The power output of the reactor is adjusted by controlling how many neutrons are able to create more fissions. Control rods that are made of a neutron poison are used to absorb neutrons. Absorbing more neutrons in a control rod means that there are fewer neutrons available to cause fission, so pushing the control rod deeper into the reactor will reduce the reactor’s power output, and extracting the control rod will increase it.

Control Rod Assembly: Control rod assembly, above fuel element
Some serious nuclear and radiation accidents have occurred. Nuclear power plant accidents include the Chernobyl disaster (1986), the Fukushima Daiichi nuclear disaster (2011), the Three Mile Island accident (1979), and the SL-1 accident (1961).
Nuclear safety involves the actions taken to prevent nuclear and radiation accidents or to limit their consequences. The nuclear power industry has improved the safety and performance of reactors and has proposed new safer (but generally untested) reactor designs. However, there is no guarantee that these reactors will be designed, built, and operated correctly.

Fukushima Daiichi Nuclear Disaster: Satellite image taken March 16, 2011 of the four damaged reactor buildings
Emission Topography
Positron emission tomography is a nuclear medical imaging technique that produces a three-dimensional image of processes in the body.
learning objectives
- Discuss possibility of uses of positron emission tomography with other diagnostic techniques.
Positron emission tomography (PET) is a nuclear medical imaging technique that produces a three-dimensional image or picture of functional processes in the body. The system detects pairs of gamma rays emitted indirectly by a positron-emitting radionuclide (tracer), which is introduced into the body on a biologically active molecule. Three-dimensional images of tracer concentration within the body are then constructed by computer analysis.
PET acquisition process occurs as the radioisotope undergoes positron emission decay (also known as positive beta decay), it emits a positron, an antiparticle of the electron with opposite charge. The emitted positron travels in tissue for a short distance (typically less than 1 mm, but dependent on the isotope), during which time it loses kinetic energy, until it decelerates to a point where it can interact with an electron. The encounter annihilates both electron and positron, producing a pair of annihilation (gamma) photons moving in approximately opposite directions. These are detected when they reach a scintillator in the scanning device, creating a burst of light which is detected by photomultiplier tubes or silicon avalanche photodiodes . The technique depends on simultaneous or coincident detection of the pair of photons moving in approximately opposite directions (it would be exactly opposite in their center of mass frame, but the scanner has no way to know this, and so has a built-in slight direction-error tolerance). Photons that do not arrive in temporal “pairs” (i.e. within a timing-window of a few nanoseconds) are ignored.
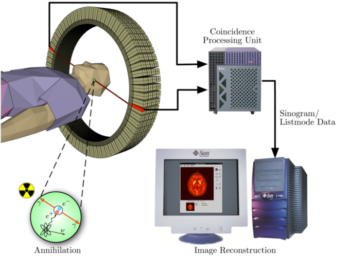
Positron Emission Tomography Acquisition Process: Schema of a PET acquisition process.
A technique much like the reconstruction of computed tomography (CT) and single-photon emission computed tomography (SPECT) data is more commonly used, although the data set collected in PET is much poorer than CT, so reconstruction techniques are more difficult.
PET scans are increasingly read alongside CT or magnetic resonance imaging (MRI) scans, with the combination giving both anatomic and metabolic information. Because PET imaging is most useful in combination with anatomical imaging, such as CT, modern PET scanners are now available with integrated high-end multi-detector-row CT scanners . Because the two scans can be performed in immediate sequence during the same session, with the patient not changing position between the two types of scans, the two sets of images are more-precisely registered, so that areas of abnormality on the PET imaging can be more perfectly correlated with anatomy on the CT images. This is very useful in showing detailed views of moving organs or structures with higher anatomical variation, which is more common outside the brain.
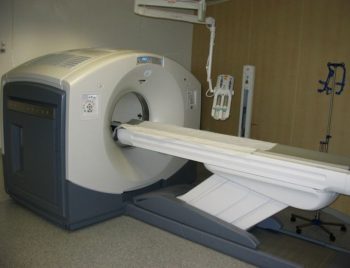
PET/CT-System: PET/CT-System with 16-slice CT; the ceiling mounted device is an injection pump for CT contrast agent.
PET scanning is non-invasive, but it does involve exposure to ionizing radiation. The total dose of radiation is significant, usually around 5–7 mSv. However, in modern practice, a combined PET/CT scan is almost always performed, and for PET/CT scanning, the radiation exposure may be substantial—around 23–26 mSv (for a 70 kg person—dose is likely to be higher for higher body weights). When compared to the classification level for radiation workers in the UK of 6 mSv, it can be seen that use of a PET scan needs proper justification.
Nuclear Weapons
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions—either fission, fusion, or a combination.
learning objectives
- Explain the difference between an “atomic” bomb and a “hydrogen” bomb, discussing their history
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission or a combination of fission and fusion. Both reactions release vast quantities of energy from relatively small amounts of matter. The first fission (i.e., “atomic”) bomb test released the same amount of energy as approximately 20,000 tons of trinitrotoluene (TNT). The first fusion (i.e., thermonuclear “hydrogen”) bomb test released the same amount of energy as approximately 10,000,000 tons of TNT.
A modern thermonuclear weapon weighing little more than 2,400 pounds (1,100 kg) can produce an explosive force comparable to the detonation of more than 1.2 million tons (1.1 million tonnes) of TNT. Thus, even a small nuclear device no larger than traditional bombs can devastate an entire city by blast, fire and radiation. Nuclear weapons are considered weapons of mass destruction, and their use and control have been a major focus of international relations policy since their inception.
Only two nuclear weapons have been used in the course of warfare, both by the United States near the end of World War II. On August 6, 1945, a uranium gun-type fission bomb code-named “Little Boy” was detonated over the Japanese city of Hiroshima. Only three days later a plutonium implosion-type fission bomb code-named “Fat Man” (as illustrated in ) was exploded over Nagasaki, Japan. The resulting mushroom cloud is shown in . The death toll from the two bombings was estimated at approximately 200,000 people—mostly civilians, and mainly from acute injuries sustained from the explosions. The role of the bombings in Japan’s surrender, and their ethical implications, remain the subject of scholarly and popular debate.

Nagasaki Atomic Bombing: The mushroom cloud of the atomic bombing of Nagasaki, Japan (August 9,1945) rose some 18 kilometers (11 mi) above the bomb’s hypocenter.

Fat Man Atomic Bomb: The first nuclear weapons were gravity bombs, such as this “Fat Man” weapon dropped on Nagasaki, Japan. They were very large and could only be delivered by heavy bomber aircraft.
Since the bombings of Hiroshima and Nagasaki, nuclear weapons have been detonated on over two thousand occasions for testing purposes and demonstrations. Only a small number of nations either possess such weapons, or are suspected of trying to acquire and/or develop them. The only countries known to have detonated nuclear weapons (and that acknowledge possessing such weapons) are, as listed chronologically by date of first test: the United States, the Soviet Union (succeeded as a nuclear power by Russia), the United Kingdom, France, the People’s Republic of China, India, Pakistan, and North Korea. In addition, it is also widely believed that Israel possesses nuclear weapons (though they have not admitted to it).
The Federation of American Scientists estimates that as of 2012, there are more than 17,000 nuclear warheads in the world, with around 4,300 considered “operational”—as in ready for use.
NMR and MRIs
Magnetic resonance imaging is a medical imaging technique used in radiology to visualize internal structures of the body in detail.
learning objectives
- Explain the difference between magnetic resonance imaging and computed tomography.
Magnetic resonance imaging (MRI), also called nuclear magnetic resonance imaging (NMRI) or magnetic resonance tomography (MRT), is a medical imaging technique used in radiology to visualize internal structures of the body in detail. MRI utilized the property of nuclear magnetic resonance (NMR) to image the nuclei of atoms inside the body.
MRI machines (as pictured in ) make use of the fact that body tissue contains a large amount of water and therefore protons (1H nuclei), which get aligned in a large magnetic field. Each water molecule has two hydrogen nuclei or protons. When a person is inside the scanner’s powerful magnetic field, the hydrogen protons in their body align with the direction of the field. A radio frequency current is briefly activated, producing a varying electromagnetic field. This electromagnetic field has just the right frequency (known as the resonance frequency) to become absorbed and then reverse the rotation of the hydrogen protons in the magnetic field.
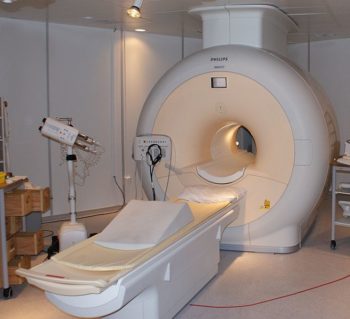
MRI Scanner: Phillips MRI scanner in Gothenburg, Sweden.
After the electromagnetic field is turned off, the rotations of the hydrogen protons return to thermodynamic equilibrium, and then realign with the static magnetic field. During this relaxation, a radio frequency signal (electromagnetic radiation in the RF range) is generated; this signal can be measured with receiver coils. Hydrogen protons in different tissues return to their equilibrium state at different relaxation rates. Images are then constructed by performing a complex mathematical analysis of the signals emitted by the hydrogen protons.
MRI shows a marked contrast between the different soft tissues of the body, making it especially useful in imaging the brain, the muscles, the heart, and cancerous tissue—as compared with other medical imaging techniques such as computed tomography (CT) or X-rays. MRI contrast agents may be injected intravenously to enhance the appearance of blood vessels, tumors or inflammation.
Unlike CT, MRI does not use ionizing radiation and is generally a very safe procedure. The strong magnetic fields and radio pulses can, however, affect metal implants (including cochlear implants and cardiac pacemakers).
Key Points
- Ionizing radiation works by damaging the DNA of exposed tissue, leading to cellular death.
- In external beam radiotherapy, shaped radiation beams are aimed from several angles of exposure to intersect at the tumor, providing a much larger absorbed dose there than in the surrounding, healthy tissue.
- In brachytherapy, a therapeutic radioisotope is injected into the body to chemically localize to the tissue that requires destruction.
- There are several ways of measuring doses of ionizing radiation: personal dosimeters, ionization chambers, and internal dosimetry.
- The distinction between absorbed dose (Gy/rad) and dose equivalent (Sv/rem) is based upon the biological effects.
- Dose is a measure of deposited dose and therefore can never decrease: removal of a radioactive source can reduce only the rate of increase of absorbed dose — never the total absorbed dose.
- The effects of ionizing radiation on human health are separated into stochastic effects (the probability of occurrence increases with dose) and deterministic effects (they reliably occur above a threshold dose, and their severity increases with dose).
- Quantitative data on the effects of ionizing radiation on human health are relatively limited compared to other medical conditions because of the low number of cases to date and because of the stochastic nature of some of the effects.
- Two pathways (external and internal) of exposure to ionizing radiation exist.
- Ionizing radiation works by damaging the DNA of exposed tissue, leading to cellular death.
- In external beam radiotherapy, shaped radiation beams are aimed from several angles of exposure to intersect at the tumor, providing a much larger absorbed dose there than in the surrounding, healthy tissue.
- In brachytherapy, a therapeutic radioisotope is injected into the body to chemically localize to the tissue that requires destruction.
- Food irradiation kills some of the microorganisms, bacteria, viruses, and insects found in food. It prolongs shelf-life in cases where pathogenic spoilage is the limiting factor.
- Food irradiation using cobalt-60 is the preferred method by most processors.
- Irradiated food does not become radioactive, since the particles that transmit radiation are not themselves radioactive.
- Radioactive tracers are used to explore the mechanism of chemical reactions by tracing the path that the radioisotope follows from reactants to products.
- The radioactive isotope can be present in low concentration and its presence still detected by sensitive radiation detectors.
- All the commonly used radioisotopes have short half-lives, do not occur in nature, and are produced through nuclear reactions.
- The fusion of lighter elements releases energy.
- Matter is not conserved during fusion reactions.
- Fusion reactions power the stars and produce virtually all elements in a process called nucleosynthesis.
- Nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts, releasing a very large amount of energy.
- Nuclear chain reactions can be controlled using neutron poisons and neutron moderators.
- Although the nuclear power industry has improved the safety and performance of reactors and has proposed new, safer reactor designs, there is no guarantee that serious nuclear accidents will not occur.
- PET scanning utilizes detection of gamma rays emitted indirectly by a positron-emitting radionuclide (tracer), which is introduced into the body on a biologically active molecule.
- PET scans are increasingly read alongside CT or magnetic resonance imaging (MRI) scans, with the combination giving both anatomic and metabolic information.
- PET scanning is non-invasive, but it does involve exposure to ionizing radiation.
- Nuclear weapons utilize either fission (“atomic” bomb) or combination of fission and fusion (“hydrogen” bomb).
- Nuclear weapons are considered weapons of mass destruction.
- The use and control of nuclear weapons is a major focus of international relations policy since their first use.
- MRI makes use of the property of nuclear magnetic resonance to image nuclei of atoms inside the body.
- MRI provides good contrast between the different soft tissues of the body (making it especially useful in imaging the brain, the muscles, the heart, and cancerous tissue).
- Although MRI uses non-ionizing radiation, the strong magnetic fields and radio pulses can affect metal implants, including cochlear implants and cardiac pacemakers.
Key Terms
- external beam therapy: Radiotherapy that directs the radiation at the tumour from outside the body.
- ionizing radiation: high-energy radiation that is capable of causing ionization in substances through which it passes; also includes high-energy particles
- brachytherapy: Radiotherapy using radioactive sources positioned within (or close to) the treatment volume.
- diode: an electronic device that allows current to flow in one direction only; a valve
- dosimeter: A dosimeter is a device used to measure a dose of ionizing radiation. These normally take the form of either optically stimulated luminescence (OSL), photographic-film, thermoluminescent (TLD), or electronic personal dosimeters (PDM).
- gamma ray: A very high frequency (and therefore very high energy) electromagnetic radiation emitted as a consequence of radioactivity.
- x-ray: Short-wavelength electromagnetic radiation usually produced by bombarding a metal target in a vacuum. Used to create images of the internal structure of objects; this is possible because x-rays pass through most objects and can expose photographic film
- radioactive tracer: a radioactive isotope that, when injected into a chemically similar substance, or artificially attached to a biological or physical system, can be traced by radiation detection devices
- isotope: any of two or more forms of an element where the atoms have the same number of protons but a different number of neutrons within their nuclei. As a consequence, atoms for the same isotope will have the same atomic number but different mass numbers (atomic weights)
- radioactive decay: any of several processes by which unstable nuclei emit subatomic particles and/or ionizing radiation and disintegrate into one or more smaller nuclei
- nucleosynthesis: any of several processes that lead to the synthesis of heavier atomic nuclei
- fusion: A nuclear reaction in which nuclei combine to form more massive nuclei with the concomitant release of energy.
- control rod: any of a number of steel tubes, containing boron or another neutron absorber, that is inserted into the core of a nuclear reactor in order to control its rate of reaction
- nuclear reactor: any device in which a controlled chain reaction is maintained for the purpose of creating heat (for power generation) or for creating neutrons and other fission products for experimental, medical, or other purposes
- fission: The process of splitting the nucleus of an atom into smaller particles; nuclear fission.
- tracer: A chemical used to track the progress or history of a natural process.
- positron: The antimatter equivalent of an electron, having the same mass but a positive charge.
- tomography: Imaging by sections or sectioning.
- warfare: The waging of war or armed conflict against an enemy.
- computed tomography: (CT) – A form of radiography which uses computer software to create images, or slices, at various planes of depth from images taken around a body or volume of interest.
- nuclear magnetic resonance: (NMRI) – The absorption of electromagnetic radiation (radio waves), at a specific frequency, by an atomic nucleus placed in a strong magnetic field; used in spectroscopy and in magnetic resonance imaging.
- magnetic resonance imaging: Commonly referred to as MRI; a technique that uses nuclear magnetic resonance to form cross sectional images of the human body for diagnostic purposes.
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- ionizing radiation. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/ionizing_radiation. License: CC BY-SA: Attribution-ShareAlike
- Nuclear medicine. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Nuclear_medicine. License: CC BY-SA: Attribution-ShareAlike
- Brachytherapy. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Brachytherapy. License: CC BY-SA: Attribution-ShareAlike
- Radiation therapy. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Radiation_therapy. License: CC BY-SA: Attribution-ShareAlike
- External beam radiotherapy. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Externa...m_radiotherapy. License: CC BY-SA: Attribution-ShareAlike
- external beam therapy. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/externa...beam%20therapy. License: CC BY-SA: Attribution-ShareAlike
- ionizing radiation. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/ionizing_radiation. License: CC BY-SA: Attribution-ShareAlike
- dosimeter. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/dosimeter. License: CC BY-SA: Attribution-ShareAlike
- Dosimetry. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Dosimetry. License: CC BY-SA: Attribution-ShareAlike
- diode. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/diode. License: CC BY-SA: Attribution-ShareAlike
- Dosimeter.gif. Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi.../Dosimeter.gif. License: CC BY-SA: Attribution-ShareAlike
- Ion-Chamber-Dosimeter-SAAP.jpg. Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi...meter-SAAP.jpg. License: CC BY-SA: Attribution-ShareAlike
- ionizing radiation. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/ionizing_radiation. License: CC BY-SA: Attribution-ShareAlike
- Biological effects of ionizing radiation. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Biologi...zing_radiation. License: CC BY-SA: Attribution-ShareAlike
- Radium Girls. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Radium_Girls. License: CC BY-SA: Attribution-ShareAlike
- /761px-USRadiumGirls-Argonne1,ca1922-23-150dpi.jpg. Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi...-23-150dpi.jpg. License: CC BY-SA: Attribution-ShareAlike
- Nuclear medicine. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Nuclear_medicine. License: CC BY-SA: Attribution-ShareAlike
- Brachytherapy. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Brachytherapy. License: CC BY-SA: Attribution-ShareAlike
- Radiation therapy. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Radiation_therapy. License: CC BY-SA: Attribution-ShareAlike
- External beam radiotherapy. Located at: en.Wikipedia.org/wiki/Externa...m_radiotherapy. License: CC BY-SA: Attribution-ShareAlike
- Radiation_therapy.jpg. Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi...on_therapy.jpg. License: CC BY-SA: Attribution-ShareAlike
- Clinical_applications_of_brachytherapy.jpg. Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi...chytherapy.jpg. License: CC BY-SA: Attribution-ShareAlike
- Food irradiation. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Food_irradiation. License: CC BY-SA: Attribution-ShareAlike
- gamma ray. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/gamma_ray. License: CC BY-SA: Attribution-ShareAlike
- x-ray. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/x-ray. License: CC BY-SA: Attribution-ShareAlike
- ionizing radiation. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/ionizing_radiation. License: CC BY-SA: Attribution-ShareAlike
- Radura-Symbol.svg.png. Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi...Symbol.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Radoactive tracer. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Radioactive_tracer. License: CC BY-SA: Attribution-ShareAlike
- radioactive decay. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/radioactive_decay. License: CC BY-SA: Attribution-ShareAlike
- isotope. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/isotope. License: CC BY-SA: Attribution-ShareAlike
- Geiger_counter_2.jpg. Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi..._counter_2.jpg. License: CC BY-SA: Attribution-ShareAlike
- Lead_container_for_nuclear_medications.jpg. Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi...edications.jpg. License: CC BY-SA: Attribution-ShareAlike
- nucleosynthesis. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/nucleosynthesis. License: CC BY-SA: Attribution-ShareAlike
- Nuclear fusion. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Nuclear_fusion. License: CC BY-SA: Attribution-ShareAlike
- fusion. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/fusion. License: CC BY-SA: Attribution-ShareAlike
- Deuterium-tritium_fusion.svg.png. Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi...fusion.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Nuclear reactors. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Nuclear_reactors. License: CC BY-SA: Attribution-ShareAlike
- Nuclear fission. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Nuclear_fission. License: CC BY-SA: Attribution-ShareAlike
- fission. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/fission. License: CC BY-SA: Attribution-ShareAlike
- Atomic reactor. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Atomic_reactor. License: CC BY-SA: Attribution-ShareAlike
- nuclear reactor. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/nuclear_reactor. License: CC BY-SA: Attribution-ShareAlike
- control rod. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/control_rod. License: CC BY-SA: Attribution-ShareAlike
- PWR_control_rod_assembly.jpg. Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi...od_assemby.jpg. License: CC BY-SA: Attribution-ShareAlike
- Nuclear_fission.svg.png. Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi...ission.svg.png. License: CC BY-SA: Attribution-ShareAlike
- Fukushima_I_by_Digital_Globe.jpg. Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi...ital_Globe.jpg. License: CC BY-SA: Attribution-ShareAlike
- Tomography. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Tomography. License: CC BY-SA: Attribution-ShareAlike
- Positron emission tomography. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Positro...ion_tomography. License: CC BY-SA: Attribution-ShareAlike
- positron. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/positron. License: CC BY-SA: Attribution-ShareAlike
- tracer. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/tracer. License: CC BY-SA: Attribution-ShareAlike
- PET-schema.png. Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi...PET-schema.png. License: CC BY-SA: Attribution-ShareAlike
- Nuclear weapon. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Nuclear_weapon. License: CC BY-SA: Attribution-ShareAlike
- Nagasakibomb.jpg. Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi...gasakibomb.jpg. License: CC BY-SA: Attribution-ShareAlike
- Magnetic resonance imaging. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/Magneti...onance_imaging. License: CC BY-SA: Attribution-ShareAlike
- magnetic resonance imagine. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/magnet...onance_imaging. License: CC BY-SA: Attribution-ShareAlike
- nuclear magnetic resonance. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/nuclea...etic_resonance. License: CC BY-SA: Attribution-ShareAlike
- computed tomography. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/computed_tomography. License: CC BY-SA: Attribution-ShareAlike
- MRI-Philips.JPG. Provided by: Wikimedia. Located at: upload.wikimedia.org/wikipedi...RI-Philips.JPG. License: CC BY-SA: Attribution-ShareAlike

