3.5: Stability of Nuclei
( \newcommand{\kernel}{\mathrm{null}\,}\)
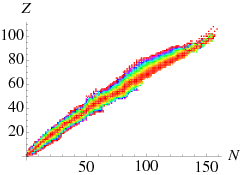
In Figure 3.5.1 we have color coded the nuclei of a given mass A=N+Z by their mass, red for those of lowest mass through to magenta for those of highest mass. We can see that typically the nuclei that are most stable for fixed A have more neutrons than protons, more so for large A increases than for low A. This is the “neutron excess”.
β decay
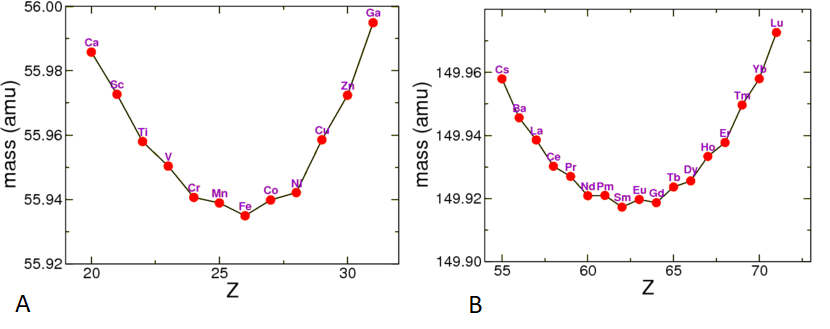
If we look at the mass of nuclides with fixed nucleon number A (i.e., roughly perpendicular cuts through the valley of stability in Figure 3.5.1), we can see that the masses vary strongly,
It is known that a free neutron is not a stable particle, it actually decays by emission of an electron and an antineutrino,
n \rightarrow p + e^-+\bar{\nu}_e. \nonumber
The reason that this reaction can take place is that it is endothermic,
m_n c^2 > m_p c^2 + m_e c^2. \nonumber
Here we assume that the neutrino has no mass.
The degree of allowance of such a reaction is usually expressed in a Q value, the amount of energy released in such a reaction,
\begin{align} Q &=m_n c^2 - m_p c^2 - m_e c^2 \\[5pt] &=939.6-938.3-0.5=0.8\text{ MeV}. \end{align} \nonumber
Generically it is found that two reaction may take place, depending on the balance of masses. Either a neutron “\beta decays” as sketched above, or we have the inverse reaction
p \rightarrow n + e^++{\nu}_e. \nonumber
For historical reason the electron or positron emitted in such a process is called a \beta particle. Thus in \beta^- decay of a nucleus, a nucleus of Z protons and N neutrons turns into one of Z+1 protons and N-1 neutrons (moving towards the right in Figure \PageIndex{2A}. In \beta^+ decay the nucleus moves to the left. Since in that figure I am using atomic masses, the Q factor is
\begin{aligned} Q_{\beta^-}&= M(A,Z)c^2-M(A,Z+1)c^2,\nonumber\\ Q_{\beta^-}&= M(A,Z)c^2-M(A,Z-1)c^2-2m_e c^2.\end{aligned} \nonumber
The double electron mass contribution in this last equation because the atom looses one electron, as well as emits a positron with has the same mass as the electron.
In similar ways we can study the fact whether reactions where a single nucleon (neutron or proton) is emitted, as well as those where more complicated objects, such as Helium nuclei (\alpha particles) are emitted. I shall return to such processed later, but let us note the Q values,
\begin{aligned} \text{neutron emission} && Q&=[M(A,Z)-M(A-1,Z)-m_n]c^2, \nonumber\\ \text{proton emission} && Q&=[M(A,Z)-M(A-1,Z-1)-M(1,1)]c^2, \nonumber\\ \text{$\alpha$ emission} && Q&=[M(A,Z)-M(A-4,Z-2)-M(4,2)]c^2, \nonumber\\ \text{break up} && Q&=[M(A,Z)-M(A-A_1,Z-Z_1)-M(A_1,Z_1)]c^2.\end{aligned} \nonumber


