2.13: Some Concepts in Thermochemistry
( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand\Dalpha
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[1], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Dbeta
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[2], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Dgamma
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[3], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Ddelta
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[4], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Depsilon
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[5], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Dvarepsilon
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[6], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Dzeta
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[7], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Deta
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[8], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Dtheta
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[9], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Dvartheta
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[10], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Diota
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[11], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Dkappa
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[12], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Dlambda
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[13], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Dvarpi
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[14], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\DGamma
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[15], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\DDelta
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[16], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\DTheta
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[17], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Vmu
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[18], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Vnu
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[19], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Vxi
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[20], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Vom
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[21], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Vpi
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[22], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Vvarpi
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[23], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Vrho
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[24], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Vvarrho
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[25], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Vsigma
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[26], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Vvarsigma
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[27], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Vtau
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[28], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Vupsilon
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[29], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Vphi
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[30], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Vvarphi
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[31], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Vchi
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[32], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Vpsi
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[33], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\Vomega
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[34], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\VGamma
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[35], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\( \newcommand\VDelta
Callstack:
at (Template:MathJaxArovas), /content/body/div/p[1]/span[36], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\newcommand\BI{\mib I}}
\)
\newcommand\Bchi{\mib\chi}
\newcommand\Bpsi{\mib\psi}
\newcommand\Bomega{\mib\omega}
\newcommand\BGamma{\mib\Gamma}
\newcommand\BDelta{\mib\Delta}
\newcommand\BTheta{\mib\Theta}
\newcommand\BLambda{\mib\Lambda}
\newcommand\BXi{\mib\Xi}
\newcommand\BPi{\mib\Pi}
\newcommand\BSigma{\mib\Sigma}
\newcommand\BUps{\mib\Upsilon}
\newcommand\BPhi{\mib\Phi}
\newcommand\BPsi{\mib\Psi}
\newcommand\BOmega{\mib\Omega}
\newcommand\Bxhi{\raise.35ex\hbox{$\Bchi$}}
\newcommand\RGamma{ \Gamma}
\newcommand\RDelta{ \Delta}
\newcommand\RTheta{ \Theta}
\newcommand\RLambda{ \Lambda}
\newcommand\RXi{ \Xi}
\newcommand\RPi{ \Pi}
\newcommand\RSigma{ \Sigma}
\newcommand\RUps{ \Upsilon}
\newcommand\RPhi{ \Phi}
\newcommand\RPsi{ \Psi}
\newcommand\ROmega{ \Omega}
\newcommand\RA{ A}
\newcommand\RB{ B}
\newcommand\RC{ C}
\newcommand\RD{ D}
\newcommand\RE{ E}
\newcommand\RF{ F}
\newcommand\RG{ G}
\newcommand\RH{ H}
\newcommand\RI{ I}
\newcommand\RJ{ J}
\newcommand\RK{ K}
\newcommand\RL{ L}
\newcommand { M}
\newcommand\RN{ N}
\newcommand\RO{ O}
\newcommand\RP{ P}
\newcommand\RQ{ Q}
\newcommand\RR{ R}
\newcommand\RS{ S}
\newcommand\RT{ T}
\newcommand\RU{ U}
\newcommand\RV{ V}
\newcommand\RW{ W}
\newcommand\RX{ X}
\newcommand\RY{ Y}
\newcommand\RZ{ Z}
\newcommand\Ra{ a}
\newcommand\Rb{ b}
\newcommand\Rc{ c}
\newcommand\Rd{ d}
\newcommand\Re{ e}
\newcommand\Rf{ f}
\newcommand\Rg{ g}
\newcommand\Rh{ h}
\newcommand\Ri{ i}
\newcommand\Rj{ j}
\newcommand\Rk{ k}
\newcommand\Rl{ l}
\newcommand { m}
\newcommand\Rn{ n}
\newcommand\Ro{ o}
\newcommand\Rp{ p}
\newcommand\Rq{ q}
\newcommand\Rr{ r}
\newcommand\Rs{ s}
\newcommand\Rt{ t}
\newcommand\Ru{ u}
\newcommand\Rv{ v}
\newcommand\Rw{ w}
\newcommand\Rx{ x}
\newcommand\Ry{ y}
\newcommand\Rz{ z}
\newcommand\BBA{\boldsymbol\RA}
\newcommand\BBB{\boldsymbol\RB}
\newcommand\BBC{\boldsymbol\RC}
\newcommand\BBD{\boldsymbol\RD}
\newcommand\BBE{\boldsymbol\RE}
\newcommand\BBF{\boldsymbol\RF}
\newcommand\BBG{\boldsymbol\RG}
\newcommand\BBH{\boldsymbol\RH}
\newcommand\BBI{\boldsymbol\RI}
\newcommand\BBJ{\boldsymbol\RJ}
\newcommand\BBK{\boldsymbol\RK}
\newcommand\BBL{\boldsymbol\RL}
\newcommand\BBM{\boldsymbol }
\newcommand\BBN{\boldsymbol\RN}
\newcommand\BBO{\boldsymbol\RO}
\newcommand\BBP{\boldsymbol\RP}
\newcommand\BBQ{\boldsymbol\RQ}
\newcommand\BBR{\boldsymbol\RR}
\newcommand\BBS{\boldsymbol\RS}
\newcommand\BBT{\boldsymbol\RT}
\newcommand\BBU{\boldsymbol\RU}
\newcommand\BBV{\boldsymbol\RV}
\newcommand\BBW{\boldsymbol\RW}
\newcommand\BBX{\boldsymbol\RX}
\newcommand\BBY{\boldsymbol\RY}
\newcommand\BBZ{\boldsymbol\RZ}
\newcommand\BBa{\boldsymbol\Ra}
\newcommand\BBb{\boldsymbol\Rb}
\newcommand\BBc{\boldsymbol\Rc}
\newcommand\BBd{\boldsymbol\Rd}
\newcommand\BBe{\boldsymbol\Re}
\newcommand\BBf{\boldsymbol\Rf}
\newcommand\BBg{\boldsymbol\Rg}
\newcommand\BBh{\boldsymbol\Rh}\}
\newcommand\BBi{\boldsymbol\Ri}
\newcommand\BBj{\boldsymbol\Rj}
\newcommand\BBk{\boldsymbol\Rk}
\newcommand\BBl{boldsymbol\Rl}
\newcommand\BBm{\boldsymbol }
\newcommand\BBn{\boldsymbol\Rn}
\newcommand\BBo{\boldsymbol\Ro}
\newcommand\BBp{\boldsymbol\Rp}
\newcommand\BBq{\boldsymbol\Rq}
\newcommand\BBr{\boldsymbol\Rr}
\newcommand\BBs{\boldsymbol\Rs}
\newcommand\BBt{\boldsymbol\Rt}
\newcommand\BBu{\boldsymbol\Ru}
\newcommand\BBv{\boldsymbol\Rv}
\newcommand\BBw{\boldsymbol\Rw}
\newcommand\BBx{\boldsymbol\Rx}
\newcommand\BBy{\boldsymbol\Ry}
\newcommand\BBz{\boldsymbol\Rz}
\( \newcommand\tcb{\textcolor{blue}\)
\( \newcommand\tcr{\textcolor{red}\)
\newcommand\bnabla{\boldsymbol{\nabla}}
\newcommand\Bell{\boldsymbol\ell}
\newcommand\dbar{\,{\mathchar'26\mkern-12mu d}}
\newcommand\ns{^\vphantom{*}}
\newcommand\uar{\uparrow}
\newcommand\dar{\downarrow}
\newcommand\impi{\int\limits_{-\infty}^{\infty}\!\!}
\newcommand\izpi{\int\limits_{0}^{\infty}\!\!}
\newcommand\etc{\it etc.\/}
\newcommand\etal{\it et al.\/}
\newcommand\opcit{\it op. cit.\/}
\newcommand\ie{\it i.e.\/}
\newcommand\Ie{\it I.e.\/}
\newcommand\viz{\it viz.\/}
\newcommand\eg{\it e.g.\/}
\newcommand\Eg{\it E.g.\/}
\newcommand\dbar{\,{\mathchar'26\mkern-12mu d}}
\def\sss#1{\scriptscriptstyle #1}
\def\ss#1{\scriptstyle #1}
\def\ssr#1{\scriptstyle #1}
\def\ssf#1{\scriptstyle #1}
\newcommand\NA{N_{\ssr{\!A}}}
\newcommand\lala{\langle\!\langle}
\newcommand\rara{\rangle\!\rangle}
\newcommand\blan{\big\langle}
\newcommand\bran{\big\rangle}
\newcommand\Blan{\Big\langle}
\newcommand\Bran{\Big\rangle}
\newcommand\intl{\int\limits}
\newcommand\half{\frac{1}{2}}
\newcommand\third{\frac{1}{3}}
\newcommand\fourth{\frac{1}{4}}
\newcommand\eighth{\frac{1}{8}}
\newcommand\uar{\uparrow}
\newcommand\dar{\downarrow}
\newcommand\undertext#1{$\underline{\hbox{#1}}$}
\newcommand\Tra{\mathop{\textsf{Tr}}\,}
\newcommand\det{\mathop{\textsf{det}}\,}
\def\tket#1{| #1 \rangle}
\def\tbra#1{\langle #1|}
\def\tbraket#1#2{\langle #1 | #2 \rangle}
\def\texpect#1#2#3{\langle #1 | #2 | #3 \rangle}
\def\sket#1{| \, #1 \, \rangle}
\def\sbra#1{\langle \, #1 \, |}
\def\sbraket#1#2{\langle \, #1 \, | \, #2 \, \rangle}
\def\sexpect#1#2#3{\langle \, #1 \, | \, #2 \, | \, #3 \, \rangle}
\def\ket#1{\big| \, #1\, \big\rangle}
\def\bra#1{\big\langle \, #1 \, \big|}
\def\braket#1#2{\big\langle \, #1\, \big| \,#2 \,\big\rangle}
\def\expect#1#2#3{\big\langle\, #1\, \big|\, #2\, \big| \,#3\, \big\rangle}
\newcommand\pz{\partial}
\newcommand\pzb{\bar{\partial}}
\newcommand\svph{\vphantom{\int}}
\newcommand\vph{\vphantom{\sum_i}}
\newcommand\bvph{\vphantom{\sum_N^N}}
\newcommand\nd{^{\vphantom{\dagger}}}
\newcommand\ns{^{\vphantom{*}}}
\newcommand\yd{^\dagger}
\newcommand\zb{\bar z}
\newcommand\zdot{\dot z}
\newcommand\zbdot{\dot{\bar z}}
\newcommand\kB{k_{\sss{B}}}
\newcommand\kT{k_{\sss{B}}T}
\newcommand\gtau{g_\tau}
\newcommand\Htil{\tilde H}
\newcommand\pairo{(\phi\nd_0,J\nd_0)}
\newcommand\pairm{(\phi\nd_0,J)}
\newcommand\pairob{(\Bphi\nd_0,\BJ\nd_0)}
\newcommand\pairmb{(\Bphi\nd_0,\BJ)}
\newcommand\pair{(\phi,J)}
\newcommand\Hz{H\nd_0}
\newcommand\Ho{H\nd_1}
\newcommand\Htz{\Htil\nd_0}
\newcommand\Hto{\Htil\nd_1}
\newcommand\oc{\omega_\Rc}
\newcommand \gtwid{\approx}
\newcommand\index{\textsf{ind}}
\newcommand\csch{\,{ csch\,}}
\newcommand\ctnh{\,{ ctnh\,}}
\newcommand\ctn{\,{ ctn\,}}
\newcommand\sgn{\,{ sgn\,}}
\def\tmapright#1{\xrightarrow \limits^{#1}}
\def\bmapright#1{\xrightarrow\limits_{#1}}
\newcommand\hfb{\hfill\break}
\newcommand\Rep{\textsf{Re}\,}
\newcommand\Imp{\textsf{Im}\,}
\newcommand\ncdot{\!\cdot\!}
\def\tmapright#1{ \smash{\mathop{\hbox to 35pt{\rightarrowfill}}\limits^{#1}}\ }
\def\bmapright#1{ \smash{\mathop{\hbox to 35pt{\rightarrowfill}}\limits_{#1}}\ }
\newcommand\bsqcap{\mbox{\boldmath{$\sqcap$}}}
\def\spabc#1#2#3{\big({\pz #1\over\pz #2}\big)\ns_{\!#3}}
\def\qabc#1#2#3{\pz^2\! #1\over\pz #2\,\pz #3}
\def\rabc#1#2#3#4{(\pz #1,\pz #2)\over (\pz #3,\pz #4)}
\newcommand\subA{\ns_\ssr{A}}
\newcommand\subB{\ns_\ssr{B}}
\newcommand\subC{\ns_\ssr{C}}
\newcommand\subD{\ns_\ssr{D}}
\newcommand\subAB{\ns_\ssr{AB}}
\newcommand\subBC{\ns_\ssr{BC}}
\newcommand\subCD{\ns_\ssr{CD}}
\newcommand\subDA{\ns_\ssr{DA}}
\def\lmapright#1{\ \ \smash{\mathop{\hbox to 55pt{\rightarrowfill}}\limits^{#1}}\ \ }
\def\enth#1{\RDelta {\textsf H}^0_\Rf[{ #1}]}
\newcommand\longrightleftharpoons{ \mathop{\vcenter{\hbox{\ooalign{\raise1pt\hbox{$\longrightharpoonup\joinrel$}\crcr \lower1pt\hbox{$\longleftharpoondown\joinrel$}}}}}}
\newcommand\longrightharpoonup{\relbar\joinrel\rightharpoonup}
\newcommand\longleftharpoondown{\leftharpoondown\joinrel\relbar}
\newcommand\cds{\,\bullet\,}
\newcommand\ccs{\,\circ\,}
\newcommand\nsub{_{\vphantom{\dagger}}}
\newcommand\rhohat{\hat\rho}
\newcommand\vrhhat{\hat\vrh}
\newcommand\impi{\int\limits_{-\infty}^\infty\!\!\!}
\newcommand\brangle{\big\rangle}
\newcommand\blangle{\big\langle}
\newcommand\vet{\tilde\ve}
\newcommand\zbar{\bar z}
\newcommand\ftil{\tilde f}
\newcommand\XBE{\RXi\ns_\ssr{BE}}
\newcommand\XFD{\RXi\ns_\ssr{FD}}
\newcommand\OBE{\Omega\ns_\ssr{BE}}
\newcommand\OFD{\Omega\ns_\ssr{FD}}
\newcommand\veF{\ve\ns_\RF}
\newcommand\kF{k\ns_\RF}
\newcommand\kFu{k\ns_{\RF\uar}}
\newcommand\SZ{\textsf Z}} \( \newcommand\kFd{k\ns_{\RF\dar}\)
\newcommand\muB{\mu\ns_\ssr{B}}
\newcommand\mutB{\tilde\mu}\ns_\ssr{B}
\newcommand\xoN{\Bx\ns_1\,,\,\ldots\,,\,\Bx\ns_N}
\newcommand\rok{\Br\ns_1\,,\,\ldots\,,\,\Br\ns_k}
\newcommand\xhiOZ{\xhi^\ssr{OZ}}
\( \newcommand\xhihOZ
Callstack:
at (Template:MathJaxArovas), /content/body/div/span[1], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\newcommand\jhz{\HJ(0)}
\newcommand\nda{\nd_\alpha}
\newcommand\ndap{\nd_{\alpha'}}
\( \newcommand\labar
Callstack:
at (Template:MathJaxArovas), /content/body/div/span[2], line 1, column 1
at template()
at (Bookshelves/Thermodynamics_and_Statistical_Mechanics/Book:_Thermodynamics_and_Statistical_Mechanics_(Arovas)/02:_Thermodynamics/2.13:_Some_Concepts_in_Thermochemistry), /content/body/p/span, line 1, column 23
\newcommand\msa{m\ns_\ssr{A}}
\newcommand\msb{m\ns_\ssr{B}}
\newcommand\mss{m\ns_\Rs}
\newcommand\HBx{\hat\Bx}
\newcommand\HBy{\hat\By}
\newcommand\HBz{\hat\Bz}
\newcommand\thm{\theta\ns_m}
\newcommand\thp{\theta\ns_\phi}
\newcommand\mtil{\widetilde m}
\newcommand\phitil{\widetilde\phi}
\newcommand\delf{\delta\! f}
\newcommand\coll{\bigg({\pz f\over\pz t}\bigg)\nd_{\! coll}}
\newcommand\stre{\bigg({\pz f\over\pz t}\bigg)\nd_{\! str}}
\newcommand\idrp{\int\!\!{d^3\!r\,d^3\!p\over h^3}\>}
\newcommand\vbar{\bar v}
\newcommand\BCE{\mbox{\boldmath{$\CE$}}\!}
\newcommand\BCR{\mbox{\boldmath{$\CR$}}\!}
\newcommand\gla{g\nd_{\RLambda\nd}}
\newcommand\TA{T\ns_\ssr{A}}
\newcommand\TB{T\ns_\ssr{B}}
\newcommand\ncdot{\!\cdot\!}
\newcommand\NS{N\ns_{\textsf S}}
Chemical reactions and the law of mass action
Suppose we have a chemical reaction among \sigma species, written as \zeta\ns_1\,\RA\ns_1 + \zeta\ns_2\,\RA\ns_2 + \cdots + \zeta\ns_\sigma\,\RA\ns_\sigma=0\ , where \begin{aligned} \RA\ns_a & = \hbox{chemical formula}\\ \zeta\ns_a & = \hbox{stoichiometric coefficient}\ .\end{aligned} For example, we could have -3\,\RH\ns_2 - \RN\ns_2 + 2\,\RN\RH\ns_3 = 0 \qquad (3\,\RH\ns_2 + \RN\ns_2\, \rightleftharpoons 2\,\RN\RH\ns_3) for which \zeta(\RH\ns_2)=-3 \quad,\quad \zeta(\RN\ns_2)=-1 \quad,\quad \zeta(\RN\RH\ns_3)=2\ . When \zeta\ns_a>0, the corresponding \RA\ns_a is a product; when \zeta\ns_a< 0, the corresponding \RA\ns_a is a reactant. The bookkeeping of the coefficients \zeta\ns_a which ensures conservation of each individual species of atom in the reaction(s) is known as stoichiometry25
Now we ask: what are the conditions for equilibrium? At constant T and p, which is typical for many chemical reactions, the conditions are that G\big(T,p,\{N\ns_a\}\big) be a minimum. Now dG=-S\,dT + V\,dp + \sum_i\mu\ns_a \,dN\ns_a\ , so if we let the reaction go forward, we have dN\ns_a=\zeta\ns_a, and if it runs in reverse we have dN\ns_a=-\zeta\ns_a. Thus, setting dT=dp=0, we have the equilibrium condition \sum_{a=1}^\sigma\zeta\ns_a\,\mu\ns_a=0\ .
Let us investigate the consequences of this relation for ideal gases. The chemical potential of the a^\ssr{th} species is \mu\ns_a(T,p)=\kT\,\phi\ns_a(T) + \kT\ln p\ns_a\ . \label{mui} Here p\ns_a=p\,x\ns_a is the partial pressure of species a, where x\ns_a=N\ns_a/\sum_b N\ns_b the dimensionless concentration of species a. Chemists sometimes write x\ns_a=[\RA\ns_a] for the concentration of species a. In equilibrium we must have \sum_a\zeta\ns_a\Big[\ln p + \ln x\ns_a + \phi\ns_a(T)\Big]=0\ , which says \sum_a \zeta\ns_a\,\ln x\ns_a = -\sum_a\zeta\ns_a \,\ln p- \sum_a\zeta\ns_a\,\phi\ns_a(T)\ . Exponentiating, we obtain the law of mass action: \prod_a x_a^{\>\zeta\ns_a} = p^{-\sum_a\zeta\ns_a}\,\exp\!\bigg(\!\!-\sum_a\zeta\ns_a\,\phi\ns_a(T)\!\bigg)\equiv \kappa(p,T)\ . The quantity \kappa(p,T) is called the equilibrium constant. When \kappa is large, the LHS of the above equation is large. This favors maximal concentration x\ns_a for the products (\zeta\ns_a>0) and minimal concentration x\ns_a for the reactants (\zeta\ns_a<0). This means that the equation REACTANTS \rightleftharpoons PRODUCTS is shifted to the right, the products are plentiful and the reactants are scarce. When \kappa is small, the LHS is small and the reaction is shifted to the left, the reactants are plentiful and the products are scarce. Remember we are describing equilibrium conditions here. Now we observe that reactions for which \sum_a\zeta\ns_a>0 shift to the left with increasing pressure and shift to the right with decreasing pressure, while reactions for which \sum_a\zeta\ns_a>0 the situation is reversed: they shift to the right with increasing pressure and to the left with decreasing pressure. When \sum_a\zeta\ns_a=0 there is no shift upon increasing or decreasing pressure.
The rate at which the equilibrium constant changes with temperature is given by \pabc{\ln \kappa}{T}{p}=-\sum_a \zeta\ns_a\,\phi'_a(T)\ . Now from Equation [mui] we have that the enthalpy per particle for species i is \Sh\ns_a=\mu\ns_a - T\pabc{\mu\ns_a}{T}{p}\ , since \CH=G+TS and S=-\pabc{G}{T}{p}. We find \Sh\ns_a=-\kB T^2\,\phi'_a(T)\ , and thus \pabc{\ln \kappa}{T}{p}={\sum_i\zeta\ns_a\,\Sh\ns_a\over \kB T^2}={\RDelta \Sh\over \kB T^2}\ , where \RDelta \Sh is the enthalpy of the reaction, which is the heat absorbed or emitted as a result of the reaction.
When \RDelta \Sh>0 the reaction is endothermic and the yield increases with increasing T. When \RDelta \Sh<0 the reaction is exothermic and the yield decreases with increasing T.
As an example, consider the reaction \RH\ns_2 + \RI\ns_2\rightleftharpoons 2\,\RH\RI. We have \zeta(\RH\ns_2)=-1\quad,\quad \zeta(\RI\ns_2)=-1\qquad\qquad \zeta(\RH\RI)=2\ . Suppose our initial system consists of \nu^0_1 moles of \RH\ns_2, \nu^0_2=0 moles of \RI\ns_2, and \nu^0_3 moles of undissociated \RH\RI . These mole numbers determine the initial concentrations x^0_a, where x\ns_a=\nu\ns_a/\sum_b \nu\ns_b. Define \alpha\equiv {x_3^0-x\ns_3\over x\ns_3}\ , in which case we have x\ns_1=x^0_1 + \half\alpha x^0_3 \qquad,\qquad x\ns_2=\half\alpha x^0_3 \qquad,\qquad x\ns_3=(1-\alpha)\,x^0_3\ . Then the law of mass action gives {4\,(1-\alpha)^2\over \alpha(\alpha + 2r)}=\kappa\ . where r\equiv x^0_1/x^0_3= \nu^0_1/\nu^0_3. This yields a quadratic equation, which can be solved to find \alpha(\kappa,r). Note that \kappa=\kappa(T) for this reaction since \sum_a\zeta\ns_a=0. The enthalpy of this reaction is positive: \RDelta \Sh > 0.
Enthalpy of formation
Most chemical reactions take place under constant pressure. The heat Q\ns_{\Ri\Rf} associated with a given isobaric process is Q\ns_{\Ri\Rf}=\int\limits_\Ri^\Rf\!\!dE+\!\int\limits_\Ri^\Rf\!\!p\,dV=(E\ns_\Rf-E\ns_\Ri)+p\,(V\ns_\Rf-V\ns_\Ri) =\CH\ns_\Rf-\CH\ns_\Ri\ , where \CH is the enthalpy, \CH=E+pV\ . Note that the enthalpy \CH is a state function, since E is a state function and p and V are state variables. Hence, we can meaningfully speak of changes in enthalpy: \RDelta \CH=\CH\ns_\Rf-\CH\ns_\Ri. If \RDelta \CH<0 for a given reaction, we call it exothermic – this is the case when Q\ns_{\Ri\Rf}<0 and thus heat is transferred to the surroundings. Such reactions can occur spontaneously, and, in really fun cases, can produce explosions. The combustion of fuels is always exothermic. If \RDelta \CH >0, the reaction is called endothermic. Endothermic reactions require that heat be supplied in order for the reaction to proceed. Photosynthesis is an example of an endothermic reaction.
| \RDelta \CH^0_\Rf | \RDelta \CH^0_\Rf | ||||||
|---|---|---|---|---|---|---|---|
| Formula | Name | State | {kJ}/{mol} | Formula | Name | State | {kJ}/{mol} |
| Ag | Silver | crystal | 0.0 | NiSO_4 | Nickel sulfate | crystal | -872.9 |
| C | Graphite | crystal | 0.0 | Al_2O_3 | Aluminum oxide | crystal | -1657.7 |
| C | Diamond | crystal | 1.9 | Ca_3P_2O_8 | Calcium phosphate | gas | -4120.8 |
| O_3 | Ozone | gas | 142.7 | HCN | Hydrogen cyanide | liquid | 108.9 |
| H_2O | Water | liquid | -285.8 | SF_6 | Sulfur hexafluoride | gas | -1220.5 |
| H_3BO_3 | Boric acid | crystal | -1094.3 | CaF_2 | Calcium fluoride | crystal | -1228.0 |
| ZnSO_4 | Zinc sulfate | crystal | -982.8 | CaCl_2 | Calcium chloride | crystal | -795.4 |
Suppose we have two reactions A + B \tmapright {(\RDelta \CH)\ns_1} C and C+D \tmapright {(\RDelta \CH)\ns_2} E\ . Then we may write A+B+D \tmapright {(\RDelta \CH)\ns_3} E\ , with (\RDelta \CH)\ns_1+(\RDelta \CH)\ns_2=(\RDelta \CH)\ns_3\ . We can use this additivity of reaction enthalpies to define a standard molar enthalpy of formation. We first define the standard state of a pure substance at a given temperature to be its state (gas, liquid, or solid) at a pressure p=1\,bar. The standard reaction enthalpies at a given temperature are then defined to be the reaction enthalpies when the reactants and products are all in their standard states. Finally, we define the standard molar enthalpy of formation \RDelta \CH^0_\Rf(X) of a compound X at temperature T as the reaction enthalpy for the compound X to be produced by its constituents when they are in their standard state. For example, if X={SO}\ns_2, then we write \RS + \RO\ns_2 \lmapright {\RDelta \CH^0_\Rf[{SO}\ns_2]} \RS\RO\ns_2\ . The enthalpy of formation of any substance in its standard state is zero at all temperatures, by definition: \enth{O\ns_2}=\enth{He}=\enth{K}=\enth{Mn}=0,
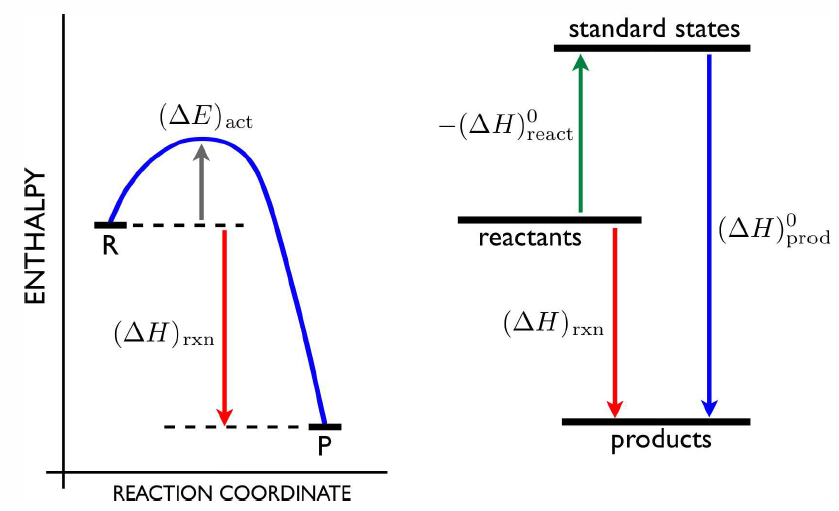
Suppose now we have a reaction a\,A + b \,B \tmapright{\RDelta \CH} c\,C + d\,D\ . To compute the reaction enthalpy \RDelta \CH, we can imagine forming the components A and B from their standard state constituents. Similarly, we can imagine doing the same for C and D. Since the number of atoms of a given kind is conserved in the process, the constituents of the reactants must be the same as those of the products, we have \RDelta \CH=-a\,\RDelta \CH^0_\Rf(A) -b\,\RDelta \CH^0_\Rf(B) + c\,\RDelta \CH^0_\Rf(C) + d\,\RDelta \CH^0_\Rf(D) \ . A list of a few enthalpies of formation is provided in table [dhtab]. Note that the reaction enthalpy is independent of the actual reaction path. That is, the difference in enthalpy between A and B is the same whether the reaction is A \longrightarrow B or A \longrightarrow X \longrightarrow (Y+Z) \longrightarrow B. This statement is known as Hess’s Law.
Note that d\CH=dE + p\,dV + V\,dp = \dbar Q + V\,dp\ , hence C\ns_p=\bigg({\dbar Q\over dT}\bigg)\nd_p = \bigg({\pz \CH\over\pz T}\bigg)\nd_p\ . We therefore have \CH(T,p,\nu)=\CH(T\ns_0,p,\nu) + \nu\!\!\int\limits_{T\ns_0}^{T}\!\!dT'\,c\ns_p(T')\ . For ideal gases, we have c\ns_p(T)=(1+\half f)\,R. For real gases, over a range of temperatures, there are small variations: c\ns_p(T)=\alpha + \beta \,T + \gamma\,T^2\ . Two examples (300\,\RK < T < 1500\,\RK, p=1\,atm): \begin{aligned} {O_2}&: & \alpha&=25.503\,{\RJ\over{mol\,K}} &,&& \beta&=13.612\times 10^{-3}\,{\RJ\over{mol\,K^2}} &,&& \gamma&=-42.553\times 10^{-7}\,{\RJ\over{mol\,K^3}} \\ {H_2O}&: & \alpha&=30.206\,{\RJ\over{mol\,K}} &,&& \beta&=9.936\times 10^{-3}\,{\RJ\over{mol\,K^2}} &,&& \gamma&=11.14\times 10^{-7}\,{\RJ\over{mol\,K^3}} \end{aligned}
If all the gaseous components in a reaction can be approximated as ideal, then we may write (\RDelta \CH)\ns_{rxn}=(\RDelta E)\ns_{rxn} + \sum_a\zeta\ns_a\,RT\ , where the subscript ‘rxn’ stands for ‘reaction’. Here (\RDelta E)\ns_{rxn} is the change in energy from reactants to products.
| enthalpy | enthalpy | enthalpy | enthalpy | ||||
| bond | ({kJ}/{mol}) | bond | ({kJ}/{mol}) | bond | ({kJ}/{mol}) | bond | ({kJ}/{mol}) |
| \RH-\RH | 436 | \RC-\RC | 348 | \RC-\RS | 259 | \RF-\RF | 155 |
| \RH-\RC | 412 | \RC=\RC | 612 | \RN-\RN | 163 | \RF-{Cl} | 254 |
| \RH-\RN | 388 | \RC\equiv\RC | 811 | \RN=\RN | 409 | {Cl}-{Br} | 219 |
| \RH-\RO | 463 | \RC-\RN | 305 | \RN\equiv\RN | 945 | {Cl}-\RI | 210 |
| \RH-\RF | 565 | \RC=\RN | 613 | \RN-\RO | 157 | {Cl}-\RS | 250 |
| \RH-{Cl} | 431 | \RC\equiv\RN | 890 | \RN-\RF | 270 | {Br}-{Br} | 193 |
| \RH-{Br} | 366 | \RC-\RO | 360 | \RN-{Cl} | 200 | {Br}-\RI | 178 |
| \RH-\RI | 299 | \RC=\RO | 743 | \RN-{Si} | 374 | {Br}-\RS | 212 |
| \RH-\RS | 338 | \RC-\RF | 484 | \RO-\RO | 146 | \RI-\RI | 151 |
| \RH-\RP | 322 | \RC-{Cl} | 338 | \RO=\RO | 497 | \RS-\RS | 264 |
| \RH-{Si} | 318 | \RC-{Br} | 276 | \RO-\RF | 185 | \RP-\RP | 172 |
| \RC-\RI | 238 | \RO-{Cl} | 203 | {Si}-{Si} | 176 |
Bond enthalpies
The enthalpy needed to break a chemical bond is called the bond enthalpy, \Sh[\,\bullet\,]. The bond enthalpy is the energy required to dissociate one mole of gaseous bonds to form gaseous atoms. A table of bond enthalpies is given in Tab. [enthtab]. Bond enthalpies are endothermic, since energy is required to break chemical bonds. Of course, the actual bond energies can depend on the location of a bond in a given molecule, and the values listed in the table reflect averages over the possible bond environment.
The bond enthalpies in Tab. [enthtab] may be used to compute reaction enthalpies. Consider, for example, the reaction 2\,\RH\ns_2(\Sg) + \RO\ns_2(\Sg)\ \longrightarrow\ 2\,\RH\ns_2\RO(\Sl). We then have, from the table, \begin{split} (\RDelta \CH)\ns_{rxn}&=2\,\Sh[\RH\!-\!\RH] + \Sh[\RO\!=\!\RO] - 4\,\Sh[\RH\!-\!\RO]\\\ &=-483\,{kJ} / {mol}\>\RO\ns_2\ . \end{split} Thus, 483 kJ of heat would be released for every two moles of \RH\ns_2\RO produced, if the \RH\ns_2\RO were in the gaseous phase. Since \RH\ns_2\RO is liquid at STP, we should also include the condensation energy of the gaseous water vapor into liquid water. At T=100^\circ\RC the latent heat of vaporization is {\tilde\ell}=2270\,\RJ/\Rg, but at T=20^\circ\RC, one has {\tilde\ell}=2450\,\RJ/\Rg, hence with M=18 we have \ell=44.1\,{kJ}/{mol}. Therefore, the heat produced by the reaction 2\,\RH\ns_2(\Sg) + \RO\ns_2(\Sg)\ \longrightleftharpoons\ 2\,\RH\ns_2\RO(\Sl) is (\RDelta \CH)\ns_{rxn}=-571.2\,{kJ}\,/\,{mol}\>\RO\ns_2. Since the reaction produces two moles of water, we conclude that the enthalpy of formation of liquid water at STP is half this value: \enth{\RH\ns_2\RO}=285.6\,{kJ}/{mol}.
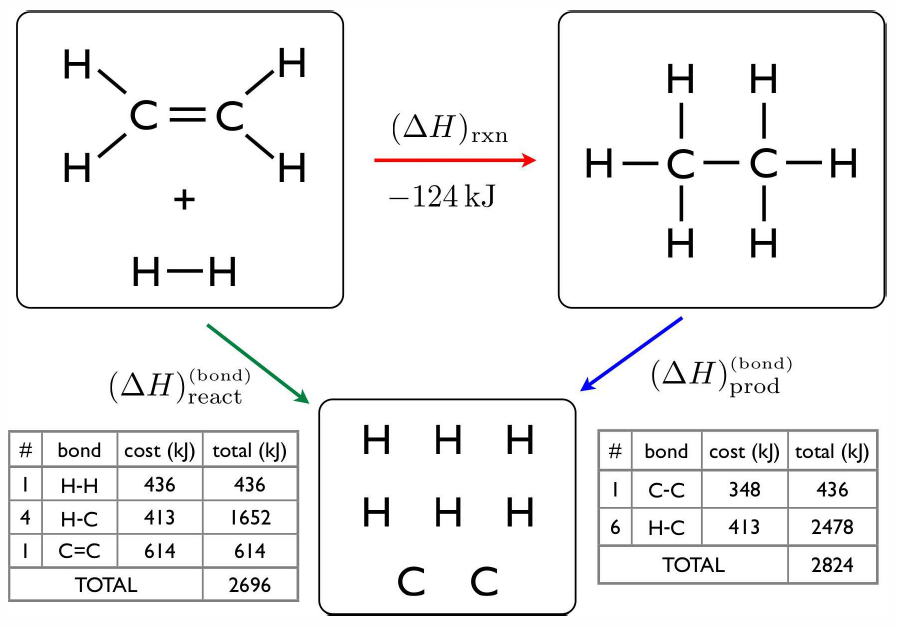
Consider next the hydrogenation of ethene (ethylene): \RC\ns_2\RH\ns_4 \ +\ \RH\ns_2\ \longrightleftharpoons\ \RC\ns_2\RH\ns_6. The product is known as ethane. The energy accounting is shown in Fig. [ethene]. To compute the enthalpies of formation of ethene and ethane from the bond enthalpies, we need one more bit of information, which is the standard enthalpy of formation of \RC(\Sg) from \RC(\Ss), since the solid is the standard state at STP. This value is \enth{\RC(\Sg)}=718\, {kJ}/{mol}. We may now write \begin{aligned} 2\,\RC(\Sg) + 4\,\RH(\Sg)\lmapright{-2260\,{kJ}} & \RC\ns_2\RH\ns_4(\Sg)\\ 2\,\RC(\Ss) \lmapright{1436\,{kJ}} & 2\,\RC(\Sg)\\ 2\,\RH\ns_2(\Sg)\lmapright{872\,{kJ}} & 4\,\RH(\Sg)\ .\end{aligned} Thus, using Hess’s law, adding up these reaction equations, we have 2\,\RC(\Ss) + 2\,\RH\ns_2(\Sg)\lmapright{48\,{kJ}} \RC\ns_2\RH\ns_4(\Sg)\ . Thus, the formation of ethene is endothermic. For ethane, \begin{aligned} 2\,\RC(\Sg) + 6\,\RH(\Sg)\lmapright{-2820\,{kJ}} & \RC\ns_2\RH\ns_6(\Sg)\\ 2\,\RC(\Ss) \lmapright{1436\,{kJ}} & 2\,\RC(\Sg)\\ 3\,\RH\ns_2(\Sg)\lmapright{1306\,{kJ}} & 6\,\RH(\Sg)\end{aligned} For ethane, 2\,\RC(\Ss) + 3\,\RH\ns_2(\Sg)\lmapright{-76\,{kJ}} \RC\ns_2\RH\ns_6(\Sg)\ , which is exothermic.


