7.11: Concept of Strong and Weak Acids and Bases
( \newcommand{\kernel}{\mathrm{null}\,}\)
Overview of Strong and Weak Acids
Acids can be classified as strong or weak based on their ability to ionize (dissociate) in water.
- Strong Acids: Completely ionize in solution. Nearly all molecules dissociate to produce hydrogen ions (H⁺).
- Weak Acids: Partially ionize in solution. Only a small fraction of molecules dissociate to produce hydrogen ions (H⁺).
Comparison Chart Acids

Explanation:
Ionization in Water:
Strong Acids: They dissociate completely in water, meaning that every molecule of the acid releases an H⁺ ion.
Example: Hydrochloric acid (HCl) dissociates as follows:

Weak Acids: They only partially dissociate in water, meaning that only some of the acid molecules release H⁺ ions.
Example: Acetic acid (CH₃COOH) dissociates as follows:
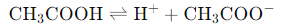
Reaction Symbols:
- Strong Acids: Represented with a single arrow (→) to indicate complete dissociation.
- Weak Acids: Represented with a double arrow (⇌) to indicate an equilibrium between dissociated and undissociated forms.
Degree of Ionization:
- Strong Acids: Nearly 100% ionization means that almost all acid molecules donate protons.
- Weak Acids: Typically, less than 5% of the acid molecules ionize, leaving most of the acid molecules undissociated in solution.
pH of Solution:
- Strong Acids: Result in a low pH (very acidic).
- Weak Acids: Result in a higher pH compared to strong acids of the same concentration.
Conductivity:
- Strong Acids: High conductivity due to a large number of ions in solution.
- Weak Acids: Lower conductivity due to fewer ions in solution.
By comparing these properties, students can better understand the behavior of strong and weak acids in aqueous solutions.
Overview of Strong and Weak Bases
Bases can be classified as strong or weak based on their ability to ionize (dissociate) in water.
- Strong Bases: Completely ionize in solution. Nearly all molecules dissociate to produce hydroxide ions (OH⁻).
- Weak Bases: Partially ionize in solution. Only a small fraction of molecules dissociate to produce hydroxide ions (OH⁻).
Comparison Chart
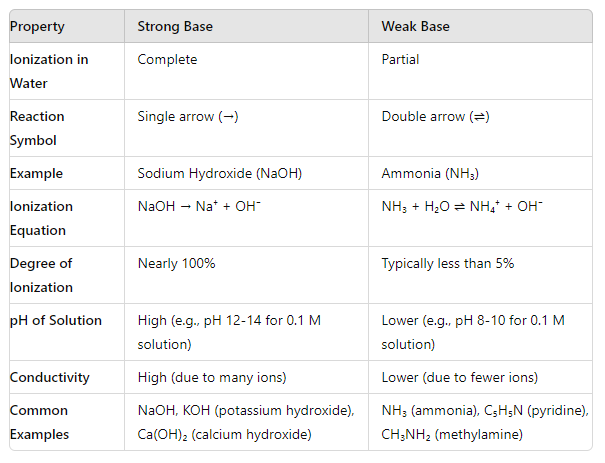
Explanation:
Ionization in Water:
- Strong Bases: They dissociate completely in water, meaning that every molecule of the base releases an OH⁻ ion.
- Example: Sodium hydroxide (NaOH) dissociates as follows:
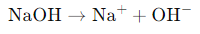
- Weak Bases: They only partially dissociate in water, meaning that only some of the base molecules release OH⁻ ions.
- Example: Ammonia (NH₃) reacts with water as follows:

Reaction Symbols:
- Strong Bases: Represented with a single arrow (→) to indicate complete dissociation.
- Weak Bases: Represented with a double arrow (⇌) to indicate an equilibrium between dissociated and undissociated forms.
Degree of Ionization:
- Strong Bases: Nearly 100% ionization means that almost all base molecules release hydroxide ions.
- Weak Bases: Typically, less than 5% of the base molecules ionize, leaving most of the base molecules undissociated in solution.
pH of Solution:
- Strong Bases: Result in a high pH (very basic).
- Weak Bases: Result in a lower pH compared to strong bases of the same concentration.
Conductivity:
- Strong Bases: High conductivity due to a large number of ions in solution.
- Weak Bases: Lower conductivity due to fewer ions in solution.
By comparing these properties, students can better understand the behavior of strong and weak bases in aqueous solutions.
Relative Strength of Acids and Bases in context of Ionization Reactions
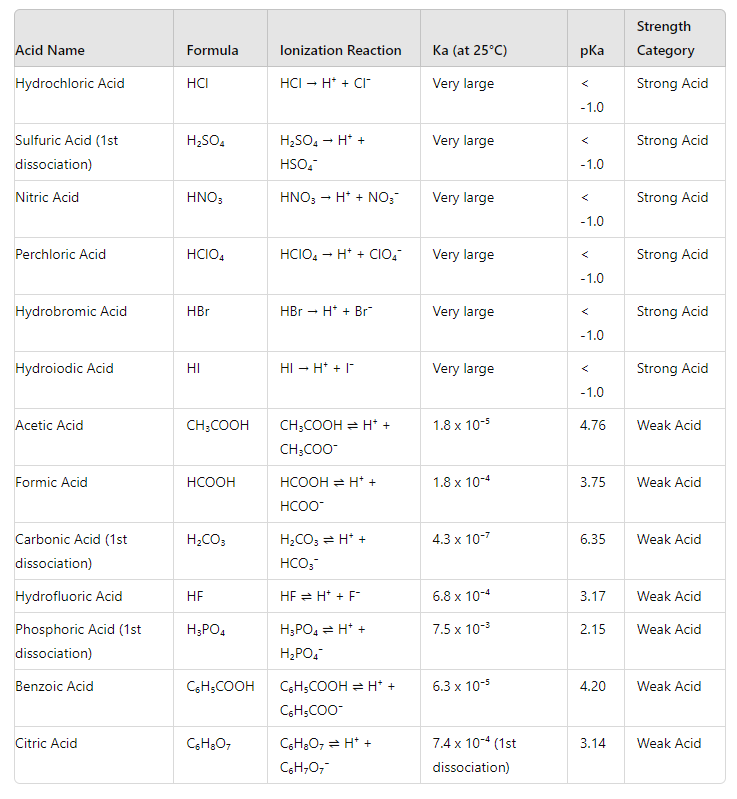

Notes
- Ka and Kb Values: The ionization constants (Ka for acids and Kb for bases) measure the extent of ionization. Higher Ka or Kb values indicate stronger acids or bases, respectively.
- pKa and pKb Values: The pKa and pKb are the negative logarithms of Ka and Kb, respectively. Lower pKa or pKb values indicate stronger acids or bases.
This table helps students visualize and understand the relative strengths of acids and bases through their ionization reactions and properties.

